Semiconductor lasers shed new light on flow cytometry
Flow cytometers that use novel semiconductor lasers are helping pharmaceutical companies better target specific proteins and develop new drugs more quickly and cost-effectively.
After 30 years as a workhorse analytical tool housed primarily in large, academic R&D centers, flow cytometers are finding their way into the labs of researchers who previously could not afford them. Thanks in part to a flurry of technology-development activity in recent years by laser makers eager to find nontelecom applications for their laser-diode products, the traditional argon laser-based instruments have been downsized to fit on a standard benchtop. This evolution has opened up new applications for flow cytometry in fields ranging from HIV and cancer research to immunology, marine biology, livestock sex selection, drug discovery, and high-throughput screening.
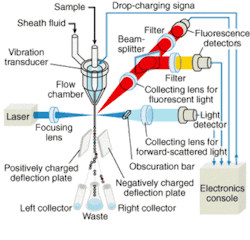
Flow cytometry—a laser-based technique for counting, examining, and sorting microscopic particles suspended in a stream of fluid—has long been recognized as a powerful tool for cell analysis (see Fig. 1). In drug discovery, it is used for precise distribution and analysis of intact cells and quantitative measurement of intracellular components (such as genes and protein lysates) and secreted proteins. Using a high-speed flow cytometer, drug-discovery scientists can simultaneously study multiple cellular properties at the single-cell level, reaching sampling rates of tens of thousands of cells per second and compound throughput rates on the order of seconds. For example, Rigel (South San Francisco, CA), a postgenomics biology company, has developed novel retroviral and gene-transfer techniques to screen millions of molecules for potential new drugs and drug targets to treat asthma, allergies, hepatitis C, cancer, rheumatoid arthritis, and other autoimmune diseases.
While flow cytometers have been a staple for large R&D labs worldwide, they have suffered from the fact that the very lasers that made them so valuable as analytical tools also made them bulky and expensive. Recent technology advances, however, have made flow cytometry more amenable to benchtop plate-based analysis and more compatible with automated drug-discovery applications. Improvements in lasers, optics, data processing, fluidics, detectors, and antibody reagents have driven prices down and put high-performance analyzers within the reach of even small to midsize labs.1 In particular, the advent of semiconductor-based 488- and 395-nm lasers has enabled Beckman Coulter, Becton Dickinson, DakoCytomation, and other manufacturers to develop more-compact systems that are less expensive but still offer much of the same analytical capabilities of their argon-laser predecessors.
"The old lasers required huge powers and water cooling, and they were huge and heavy and unstable and very expensive," said Todd Lary, group manager in product development at Beckman-Coulter (Fullerton, CA). "Cells haven't changed their size in the last 10 years, but lasers and computers certainly have. The new lasers are smaller, the signals are quieter, and they are much more efficient so they create less heat, which means we need to get rid of less heat and this takes less power. The end result is that they are smaller and more affordable, so instead of a lab having one huge instrument they can have several smaller instruments."
How it works
A typical flow cytometer comprises a light source, a flow chamber, an optical assembly, photodetectors, and processors to convert the light signals into analog electrical impulses, analog-to-digital converters, and a computer system to analyze and store acquired data (see Fig. 2). A beam of light of a single frequency is directed onto a hydrodynamically focused stream of fluid. Two detectors are aimed at the point where the stream passes through the light beam, one in line with the light beam and one perpendicular to it. Each suspended particle passing through the beam scatters the light in some way, and chemicals in the particle can be excited into emitting light at a lower frequency than the light source. This combination of scattered and fluorescent light is picked up by the two detectors; by analyzing changes in brightness and frequency at each detector it is possible to deduce various facts about the physical and chemical structure of each individual particle.
For years, flow cytometers used water-cooled gas lasers for fluorochrome excitation because of their low noise levels, stable power levels, and true Gaussian beam configurations. To reduce the size and cost of these systems, flow-cytometry manufacturers began using 30-mW air-cooled gas lasers (primarily argon-ion, HeNe, and HeCd lasers) in the 1980s; while the resulting benchtop systems, such as the FACScan from Becton Dickinson (Franklin Lakes, NJ), have been popular and are still widely used, they require relatively complex cooling systems and are limited in wavelength range.
Semiconductor lasers, though not tunable, offer several advantages for flow cytometry, including smaller size and reduced heat output. However, the technology was not powerful or robust enough, nor was the beam quality good enough, for this application until the late 1990s. In 2001, Coherent (Santa Clara, CA) introduced the first 488-nm semiconductor laser for flow cytometry, the Sapphire—a vertical-external-cavity surface-emitting laser (VECSEL) that emits up to 200 mW at 488 nm. Unlike conventional vertical-cavity lasers, the Sapphire uses 808-nm diodes to optically (versus electrically) pump a semiconductor wafer to emit a fundamental output of 976 nm that is frequency-doubled to 488 nm. According to the company, optical pumping allows higher power with excellent spatial mode.
Today the Sapphire is considered the leading semiconductor-based laser for flow cytometry, and its introduction is hailed as a paradigm shift in biomedical instrumentation. According to the company, it is 90% smaller than a comparable argon-ion laser, consumes 98% less power, and dissipates 98% less heat.
"The optically pumped design that we have is extremely robust, and most of our test lasers are now at 30,000 hours and counting, which is longer than the lifetimes of the instruments themselves," said Paul Ginouves, Ion Business Unit manager at Coherent. "These lasers are inherently robust, and scalable, the optical trains are reasonably well-developed, and you can grow the gain material to address any line you want, within reason."
Electrically vs. optically pumped
Since the introduction of the Sapphire, semiconductor-based lasers in a variety of colors and wavelengths have been introduced into flow cytometers, including red (635 nm), blue (488 nm), green (532 nm), and violet (395 to 410 nm). Some cytometers use multiple lasers to stimulate different excitation wavelengths, such as the CyAn ADP from DakoCytomation (Copenhagen, Denmark), which features three lasers to detect up to nine colors. In addition, because excitation is not limited to light in the visible spectrum, some manufacturers offer instruments configured with UV-emitting sources (370 to 385 nm) that can be used in conjunction with various dyes.
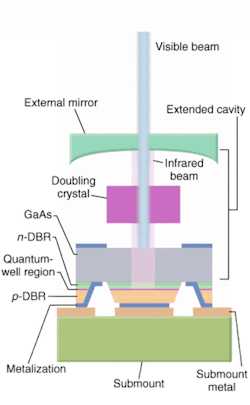
Efforts are also under way to further reduce the size and improve the performance of these semiconductor lasers and, in turn, flow cytometers. The Protera laser from Novalux (Sunnyvale, CA), for example, is an electrically pumped intracavity frequency-doubled laser that the company says further reduces cooling requirements and improves beam quality (see Fig. 3). The Protera features the company's proprietary NECSEL (Novalux Extended Cavity Surface Emitting Laser) technology, which uses a three-mirror cavity configuration to produce very high output powers in a collimated, diffraction-limited beam at wavelengths ranging from 460 to 1150 nm.
"Our target all along with this product was the air-cooled argon, so we replicated that beam," said Rick Waltonsmith, director of sales and marketing at Novalux. "The diameter, power stability, and pointing stability are all the same, and we do it for the same amount of money, with better lifetimes."
The Protera can produce 460-, 488-, and 532-nm light with very low noise and no need for external filters. Novalux is notes that its technology is less expensive than other semiconductor-based lasers.
On the systems side, Partec (Munster, Germany) introduced the CyFlow family of portable and desktop flow cytometers and cell counters in 2000. These systems are available in a several configurations using Nd:YAG and semiconductor lasers and in the CyFlow mobile laboratory, which is in use in 25 developing countries for CD4 counting and HIV monitoring.
Another company, Guava Technologies (Hayward, CA), is leveraging advances insemiconductor lasers to develop analytical instruments that are intended to revolutionize cell-culture monitoring and cell screening through the use ofmicrocapillary cytometry. The company's Guava PCA (personal cell analysis), Guava PCA-96, and Guava PCA-96 AFP systems offer turnkey operation and multiple assays in desktop configurations that are about a third of the size of conventional cytometers and less than half the price: around $40,000 versus $100,000 to $200,000 (see Fig. 4).
The heart of the PCA systems is patented, laser-based microcapillary technology capable of detecting mammalian and microbial cells and beads. Fluorescently labeled cells are aspirated into a microcapillary flow cell in which a green or blue diode laser excites 200 picoliters of sample containing cells—each cell emits signals that are individually detected by photomultipliers and a photodiode. Guava's software modules show all the relevant data and results immediately on a laptop screen. The unique fluid dynamics of the capillary flow cell provide a range of new and unique applications and eliminate the need for the sheath fluid required in conventional flow cytometry.
"Our approach is a signal departure from traditional flow cytometry," said Rajen Dalal, Guava CEO. "Our system relies heavily on new optical technologies, but it also eliminates all the hydraulics of the traditional flow cytometers and replaces them with a microcapillary tube."
Dalal sees a bright future for Guava in the broader market the PCA products are expected to penetrate, putting flow cytometry into the hands of end users who previously could not afford them. If he is right, this trend should eventually lead to the use of flow cytometers for disease assessment.
"Cells are becoming important predictive models in drug discovery, and the smaller systems will put this capability in the hands of a much larger group of end users," Dalal said. "We visualize the market dynamics as a pyramid, with the core labs at the apex and the cancer research and drug discovery labs in the middle tier. At the bottom of the triangle is the 100,000 or more cell biologists and protein analysts who don't even do cell analysis at this point because the tools don't exist for them today. We believe the new products will serve this bottom tier and that the same migration is likely to occur in the clinical diagnostics area by taking the enormous diagnostic capabilities of flow cytometry and for the first time putting it into the hands of physicians."
REFERENCE
- J. P. Roberts, The Scientist 17(9) (May 5, 2003).