OPTOFLUIDICS: ARROW-based lab on a chip sees one bioparticle at a time
Researchers at the University of California Santa Cruz (UCSC) have created fluid-filled antiresonant reflecting optical waveguides (ARROWs) on a silicon chip to form a tool that, using fluorescence-correlation spectroscopy (FCS), can detect the presence of single nanosize biological particles such as fluorescently tagged liposomes.1 The UCSC group is experienced with ARROW technology; for example, the group previously created chips with vapor-filled ARROWS for atomic spectroscopy (see www.laserfocusworld.com/articles/298368. This new optofluidic chip bolsters the capabilities of micron-size ARROWs in the lab-on-a-chip arena.
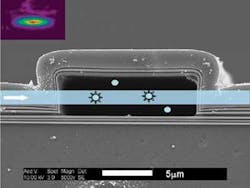
The hollow-core ARROW is fabricated around a sacrificial 5 × 12 µm core layer, with multilayer thin films of silicon dioxide and silicon nitride added via plasma-enhanced deposition; the core is then etched away (see figure). Perpendicular to the hollow-core ARROW, or at locations where the liquid-core ARROW turns a corner, solid-core ARROWs are fabricated on the chip in a separate lithographic step; the solid-core waveguides carry fluorescent-excitation light into or out of the hollow-core waveguide (which contains the liposome-bearing fluid). “The main challenges were to identify a sacrificial core material that would result in smooth top layers and could be etched out with high selectivity,” says Holger Schmidt, associate professor of electrical engineering.
The resulting chip holds a network of intersecting solid- and hollow-core ARROWs; the liquid-core ARROW is terminated by reservoirs to allow controlled fluid delivery. The optical-excitation volume at each light-introducing intersection on the experimental chip is about 85 femtoliters.
For FCS, electrodes are integrated into the device to allow electrophoresis of the nanoparticles (a voltage-dependent force that moves the particles along the waveguide, resulting from a negative electrical charge on the dye molecules inside the liposome). In the technique, particle concentration and diffusion speeds are determined by measuring the changes in fluorescence in the optical-excitation volume when a voltage is applied. Fluorescence bursts were seen; analysis of the data showed an average of 0.64 liposomes in the excitation volume, showing single-particle-detection capability. Further analysis showed that the liposomes had a narrow size distribution. The ability to control the speed of liposome drift as a function of voltage was also confirmed.
For upcoming versions of the ARROW-based lab on a chip, Schmidt envisions integrating more of the fluid-handling technology that has already been developed in microfluidics, including valves, multiple sources, and other ways to mix fluids. “We would like to incorporate additional optical elements and components such as filters, light source, and detector to eventually make a fully self-contained platform,” he says. “Another target would be to add electrical elements, as well as more control over the movement of individual molecules. We would also like to demonstrate high-sensitivity detection of clinically relevant particles such as viruses.”
REFERENCE
1. D. Yin et al., Lab Chip 2007, DOI: 10.1039/b708861b.
About the Author
John Wallace
Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.
