Laser shock peening in the medical sector: An overview
XIAOJUN SHEN
EDITOR’S NOTE: This article was reprinted from The Laser User, Issue 93, Summer 2019, Pages 20-21, with permission from the author and The Association of Laser Users (AILU); www.ailu.org.uk.
Laser shock peening (LSP) has been proved to be an innovative surface modification technique since its development in the 1970s. The technique was initially employed to resolve aviation and manufacturing problems for improving fatigue life, anticorrosion, and wear resistance. Since then, it has been used to improve the performance of aerospace components of engines. In recent years, increasing numbers of researchers have tried to find its possible value in the medical sector. This study is focused on recent research regarding the surface modification of medical-grade metals and alloys subjected to LSP.
Residual stress and fatigue
Induction of compressive residual stress is the primary goal of LSP, as this has many benefits for improving the functional properties of metallic components. One of these benefits is that it can decrease the risk of metal fracture. The effects of LSP on initiation and propagation of fatigue cracks in aluminum alloy AA2024 with a thickness of 2.0 mm have been investigated.1 This study observed a significant decrease in the fatigue crack propagation rates due to the compressive residual stress. Additionally, LSP is also used to solve the cracking and fracture problems of nickel-based alloys.
In a previous study, a team of researchers at Coventry University (Coventry, England) reported that the fatigue life of K403 nickel alloy samples subjected to three impacts of LSP were improved by 244%.2 Through analyzing the crack fracture morphology, it was found that it took more energy for the fatigue cracks to propagate in the laser shock-peened samples than in non-peened samples.
Researchers have started to use LSP to improve the fatigue life of medical implants. Initial work has shown that a flat rod of Ti6Al4V (TC4) titanium alloy conventionally used for biomedical implants can be laser-peened to induce in-plane compressive residual stress ranging from -300 to 350 MPa.3 Additionally, an increase in the fatigue load ratio was observed.
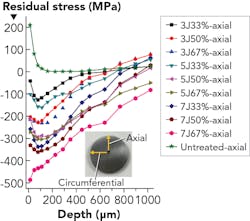
In order to investigate the residual stress distribution of TC4 hip replacements, another investigation showed a 3D hip modeled in Abaqus software to simulate the initiated cracks as the implant failed.4 By using LSP, a compressive residual stress of 600 MPa was formed in the surface, shown in both simulated models and residual stress experiments. TC4, however, is less ideal for a bioimplant than Ti-Al-67-Nb. This is because vanadium (present in TC4) is toxic when it is released into the body, while niobium is non-toxic to the human body. In a previous study by the Coventry University researchers and colleagues,5 they utilized Ti-Al-67-Nb for the first time to induce compressive residual stress of -41 to -516 MPa by applying laser energy of 3, 5, and 7 J with overlaps of 33%, 50%, and 67%, respectively. Results illustrated in FIGURE 1 show that Ti-Al-67-Nb can be laser-peened as well as the other titanium alloys previously studied by others.
Corrosion
Corrosion has been a crucial problem with orthopedic implants since metals were employed as biomaterials. For example, magnesium has excellent biocompatibility and has been widely used for fabrication of medical devices. Additionally, magnesium-made implants can greatly reduce stress shielding effects, as the density of magnesium and its elastic modulus are very close to human bones (density: 1.74 - 2g/cm3 vs. 1.8 - 2.1g/cm3; elastic modulus: 42–45 GPa vs. 40–57 GPa) compared to other traditional metal materials such as stainless steel and titanium alloys. However, magnesium was found to corrode in physiological and enriched Cl-ions solutions.6 What's more, hydrogen gas pockets were generated adjacent to the implants while magnesium alloy was corroding. Therefore, the use of magnesium was greatly limited by its poor anticorrosion capability in its further application in orthopedic implants.
As a result, much research is being dedicated to improving the anticorrosive properties of magnesium, with some success. Guo et al. explored the process capability of LSP to control the corrosion of magnesium-calcium implants by tailoring the surface integrity.7 More specifically, the importance of parameters such as overlap have been shown during the experiments—for example, high overlap with lower power density on commercial purity magnesium in simulated body solutions (Hank’s solution).8 The results showed that the corrosion rate of peened samples was at least 6X lower than unpeened samples and 66% overlap scans presented the least corrosion. Therefore, the improvement of corrosion resistance by LSP will enable metallic implants to endure longer in the human body and reduce the risk of failure caused by corrosion.
Wear
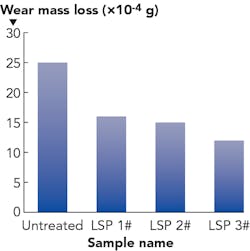
Wear resistance is another important mechanical property of medical implants. Wear failure is responsible for 13.6% of all implant failures.9 As mentioned, titanium alloy is an excellent material for manufacturing medical devices due to its superior biocompatibility and mechanical properties. However, its wear resistance ability is comparatively low and sliding wear data of titanium subjected to LSP is still scarce. In our previous work, a sliding wear experiment of Ti-6Al-7Nb subjected to LSP was carried out. The wear mass loss comparison of Ti-6Al-7Nb alloy coupons after dry wear experiments are shown in FIGURE 2. It can be seen that the wear mass loss of impacted coupons after successive laser peening impacts were much lighter than untreated coupons (25*10-4g). This decreased with the increasing impacts. Therefore, multiple LSP can effectively improve the sliding wear property of Ti-6Al-7Nb.Wettability
Wetting characteristics of biomaterial surfaces integrated with surface characteristics such as surface energy, macro-, and nano-topography play an important role in cell/protein adhesion and osseointegration (the connection between living bone and the surface of a load-bearing artificial implant). Wetting determines the biological chain interactions at the implant-tissue interface that include protein absorption to hard and soft tissue interactions, and cell interactions (adhesion, differentiation, and migration).
Caralapatti et al. recently investigated the effect of high-repetition LSP on wettability of pure magnesium.11 It was found that the surface wettability of peened magnesium samples was increased, with the contact angle being increased to 81.3°. Prabhakaran et al. came to a similar conclusion when they used LSP to peen the surface of austenitic stainless steel, which is hydrophilic in nature.12 The contact angle of unpeened samples was 34.24° and the surface roughness was also increased by LSP. The unpeened austenitic stainless steel is hydrophilic in nature. But, after one impact, the contact angle was increased to 95.75°, thereby becoming hydrophilic as a result of LSP.
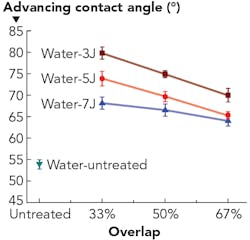
The Coventry University group's research is focused on the modification of wetting characteristics using LSP. The effects of laser energy (3, 5, and 7 J) and beam footprint overlap (33%, 50%, and 67%) of LSP were explored on Ti-6Al-7Nb alloy, quantified by using the measurement of dynamic contact. The dynamic advancing contact angle of Ti-6Al-7Nb before and after LSP wetted with distilled water is presented in FIGURE 3. The contact angle of untreated samples with distilled water is 54°. In comparison, all LSP contact angles are higher than the untreated one. The contact angle values rise with the increase of laser energy at the same overlap. Meanwhile, the contact angles decrease with the increase of overlap at the same laser energy level.Biocompatibility
At present, published literature evaluating cell behavior subject to surfaces treated by LSP is still scarce. A study on in vitro cell culture demonstrated that LSP did not compromise the cytotoxicity of AZ321B in vitro13 and still provided good strength to the part.
Creating groove architecture on the titanium surfaces using LSP can enhance the osseointegration of an implant with the host tissue.14 Osteoblast cell adhesion and differentiation on Ti-6Al-4V implants were also improved due to the modified surface morphology by LSP. Like other mesenchymal-derived cells, osteoblasts are one of the most crucial cell types during the recovery stage after the surgical implant procedure. They need anchorages to survive, proliferate, and differentiate, which LSP modified surfaces can provide.
Conclusion
Laser shock peening has a great potential to enable biomaterials to improve fatigue, corrosion, and wear resistance, and create surface ‘anchorages’ for cell attachment. This surface modification technique is very useful because it not only induces unique topography, but also induces compressive residual stresses that are highly beneficial for the end-user.
REFERENCES
1. N. Kashaev et al., Int. J. Fatigue, 98, 223–233 (2017).
2. C. Wang et al., Mater. Design, 89, 582-588 (2016).
3. S. R. Mannava et al., Int. J. Struct. Integr., 2, 1, 101–113 (Mar. 2011).
4. C. Correa et al., Mater. Design, 79, 106–114 (2015).
5. X. Shen et al., J. Alloys Compd., 801, 327–342 (2019).
6. Y. Zhang et al., Surf. Coat. Technol., 204, 3947–3953 (2010).
7. Y. Guo et al., CIRP Ann. Manuf. Technol., 61, 583–586 (2012).
8. V. K. Caralapatti et al., Opt. Laser Technol., 93, 165–174 (2017).
9. See http://bit.ly/2N3glCB.
10. X. Shen et al., Surf. Coat. Technol., 327, 101–109 (2017).
11. V. K. Caralapatti et al., Opt. Laser Technol., 88, 75–84 (2017).
12. S. Prabhakaran et al., Appl. Surf. Sci., 428, 17–30 (2018).
13. R. Zhang et al., Surf. Coat. Technol., 339, 48–56 (2018).
14. M. Khandaker et al., Int. J. Nanomed., 11, 585–595 (2016).
XIAOJUN SHEN ([email protected]) is a PhD researcher in the Faculty of Engineering, Environment and Computing at Coventry University, Coventry, England; www.coventry.ac.uk.