Phototherapy: Photobiomodulation therapy—easy to do, but difficult to get right
PRAVEEN ARANY
Light is an all-pervasive aspect of life as we know it today, from providing the ultimate cosmological yardstick to its relationship to the fundamental nature of matter. Besides our physical world, evidence for the use of light to improve human health can be traced to various ancient civilizations, such as the use of chromotherapy in Egyptian healing practices.
A more thorough understanding of light and its biological interactions has led to precise elucidation of several health benefits, such as adequate sunlight and Vitamin D metabolism for skin and bones, vision and perception by the brain, circadian rhythm, and psychological state. In medicine, the use of various light-based treatments are popular, such as blue-light phototherapy for jaundice in babies as well as Psoralen-Ultraviolet-A (PUVA) treatment for psoriasis.
Another treatment uses a combination of light and a photosensitive drug to destroy tumor cells or microbes and is termed photodynamic therapy. These treatments are based on the ability of selective absorption of light by a targeted chromophore or, in the case of cancer, a cancer-attached nanoparticle or compound that absorbs light and leads to cancer cell destruction.
In contrast to these destructive processes, the use of low-dose biophotonic light treatments to alleviate pain and inflammation or promote tissue healing and regeneration is termed photobiomodulation (PBM) therapy. The universal acceptance of the term PBM in 2015 has proven to be a major factor in its broader recognition. Previously, more than 70 different terminologies, including low-level light/laser therapy, cold laser, and photostimulation, were quite popular to describe this treatment.1
Photobiomodulation benefits
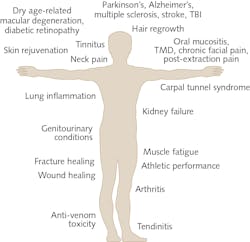
The list of clinical conditions benefiting from PBM therapy is a rather startling list, from conditions affecting the brain to tendinopathy in the foot and acute pains to chronic fatigue (see Fig. 1). Indeed, this seemingly all-encompassing approach with PBM therapy has brought the field much disrepute as a marginal, alternate approach.
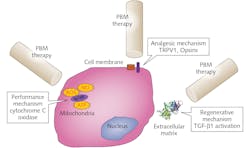
However, recent advances in our understanding of precise molecular mechanisms have provided a much-needed boost to appreciating its specific clinical benefits.2 Among these recent advances, three discrete PBM mechanisms have been described—namely, inside the cell (cytochrome C oxidase within mitochondria), at the cell membrane (photosensitive receptors and ion transporters like Opsins, TRPV1), and extracellular (activation of latent TGF-ß1).3-7 While there may be a predominant mechanism operative in a given PBM application, research is showing that there are typically several mechanisms at work creating specific biological responses noted during clinical therapy (see Fig. 2).A major problem with this field has been a lack of reproducibility and rigor of clinical benefits. Significant recent effort in the field has narrowed down on two major areas in PBM that can further improve clinical safety and efficacy of this innovative treatment. First, there are likely anatomy-specific and disease pathophysiology-based PBM mechanisms at play that will need to be thoroughly investigated. For example, pain in the knee will require a different approach than pain in the gut or shoulder. Another example is treatment for inflammatory muscle soreness will need to harness singularly discrete pathways with PBM therapy as compared to a regenerative mechanism for muscle damage and repair.
A recent paper from the Multinational Association for Supporting Care in Cancer and International society of Oral Oncology (MASCC/ISOO) outlines a quantitative prescription for PBM therapy in prevention and treatment of oral mucositis, a common side effect of chemotherapy.8 This is a major landmark for the field, as it is the first clear clinical recommendation for the use of PBM therapy in mainstream medicine medicine based on a thorough systematic review and meta-analyses of several well done, multicenter, placebo-controlled human clinical studies.
There are several other human diseases where PBM therapy is poised to make a major impact in medicine such as Alzheimer’s disease, Parkinson’s disease, stroke, traumatic brain injury (TBI), concussions, chronic wounds (diabetic, venous, or pressure), age-related macular degeneration, back pain, tendinopathy, and diabetic retinopathy, among many others.9
Quantifying light dosage
Another unique aspect of PBM therapy is the dose response. Most biological interventions follow the usual dose-escalation path with an optimum threshold dose, beyond which there is a plateau or toxicity. Photobiomodulation dosing follows a rather unique Arndt-Schultz dose curve where too-little light energy has no effect and optimal amounts evoke therapeutic responses, while further increases can result in an antagonistic effect. This has resulted in a significant number of inefficacious or poor responses in PBM clinical studies.
A key aspect of PBM dosing is the emphasis on dose delivery and clinical protocols. A significant paradigm shift in recent years has been a move from single-point-source lasers to large LED arrays to address both treatment field consistency as well as to reduce treatment time and improve consistency during treatments.
These latter LED arrays, some in whole body formats such as light beds, shower heads, body pods, and walk-in cabinets among many other types, are nothing short of a mini-revolution for the field. In contrast to the increased emphasis on digital screen times and ‘light hygiene’ with blue-light filters, a recent study that simply swapped regular lighting for PBM wavelengths in an assisted living home generated striking improvements in depression, age-related dementia, and sleep patterns.10
Despite such advances, the plethora of light devices has also created another unique challenge for the field. Besides sophisticated expensive lasers and large arrays of LED and broadband light devices, there has been a recent increase into home-made do-it-yourself (DIY) light devices that are being employed by experts and novices alike. The easy access to the light sources and striking publications have enabled inexpensive DIY sources for PBM treatments that have become very appealing. There are some remarkable stories, such as the use of a ‘light bucket’ or type of head-worn helmet that targets the brain that has shown efficacy for Parkinson’s disease (see Fig. 3).11 Nonetheless, these unproven devices raise concerns on efficacy and the potential failing of patients to seek timely professional care.A major technical limitation for the PBM field is a lack of suitable devices for further optimization of these studies. For lasers as clinical treatment devices, a laser point source could be envisioned for treatment approaches that would triangulate (two- or multiphoton) or use robotic scanners to distribute PBM dose effectively.
The photonics industry could further help spur advances in PBM by increasing the availability and decreasing the cost of light sources such as expanding the current focus from blue (±450 nm), red (±660 nm) and near-infrared (above 700 nm) PBM wavelengths. A tunable laser source with a large spot size (>10 cm) and variable irradiance (from 1 to 200 mW/cm2) is highly desirable. Innovations in LED-based PBM devices could improve the uniformity of surface treatments if a broader array of wavelengths with variable irradiance were available at lower cost. This is truly a watershed moment in the history of biophotonics, as there is tremendous potential for PBM treatments to revolutionize human health.
REFERENCES
1. J. J. Anders, R. J. Lanzafame, and P. R. Arany, Photomed. Laser Surg., 33, 4, 183–184 (2015).
2. P. R. Arany, J. Dent. Res., 95, 9, 977–984 (2016).
3. P. R. Arany et al., Sci. Trans. Med., 6, 238, 238ra269 (2014).
4. S. Buscone et al., Lasers Surg. Med., 49, 7, 705–718 (2017).
5. T. I. Karu and S. F. Kolyakov, Photomed. Laser Surg., 23, 4, 355–361 (2005).
6. Y. Wang et al., Sci. Rep., 7, 1, 7781 (2017).
7. P. D. Yim et al., Am. J. Physiol. Lung Cell Mol. Physiol., 316, 1, L82–L93 (2019).
8. Y. Zadik et al., Support. Care Cancer (2019); https://dx.doi.org/10.1007/s00520-019-04890-2.
9. M. Hennessy and M. R. Hamblin, J. Opt., 19, 1, 013003 (2017).
10. M. G. Figueiro et al., Clin. Interv. Aging, 9, 1527–1537 (2014).
11. M. Dadson, “Tasmanian red light helmet treatment for Parkinson’s disease symptoms prompts clinical trial” (2019); https://ab.co/2JHQPRS.
Praveen Arany is president of the World Association for photobiomoduLation Therapy (WALT; https://waltza.co.za) and assistant professor in the Oral Biology and Biomedical Engineering Department at the University at Buffalo, Buffalo, NY; e-mail: [email protected]; https://dental.buffalo.edu.