Trends in flow cytometry lasers call for more new wavelengths
The trend toward personalized medicine, which has been further accelerated by the ongoing COVID-19 pandemic, is fueling a need for more advanced flow cytometry instrumentation for both research and clinical applications. Specifically, so-called high-dimensional—or multiparameter—flow cytometry uses more laser wavelengths to deliver a bigger dataset and therefore measure a larger number of different cell types in a single instrument. Laser manufacturers are now meeting this requirement with new wavelengths in the UV, visible, and near-infrared (near-IR) ranges, as well as with multiwavelength laser light engines that streamline instrument development for manufacturers.
Flow cytometry basics
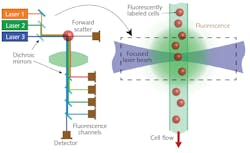
A flow cytometer is an instrument that can count the number of various cell types in a blood sample. To accomplish this, the blood sample is first concentrated and then treated with a mixture of fluorochromes—fluorescent chemicals that each preferentially bond to a specific protein target on the cell surface. After treatment, the cells are loaded in the instrument, delivering them in single file in a flow stream to the laser interaction zone. Several different-wavelength laser beams are focused on the stream (see Fig. 1), resulting in fluorescence and light scatter. Each fluorochrome fluoresces at a different wavelength, which will ultimately provide the ability to differentiate the cells.
Optics collect the fluorescence signal and separate it into wavelength bins using bandpass filters. The light signals are quantitatively measured using detectors (photomultiplier tubes or avalanche photodiodes).
Clinical instruments typically use up to four laser wavelengths and up to 10 detectors. The latest research instruments use up to nine laser wavelengths and 60 detectors. Both instrument types are fast, typically analyzing hundreds of cells per second.
Challenges of high-dimensional cytometry
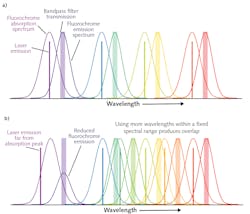
The fluorescence signal is always at a longer wavelength (Stokes-shifted) than the laser excitation (see Fig. 2). This shift allows fluorescence to be efficiently separated from the scattered laser light using a combination of bandpass and cut-off filters. The theoretically maximum signal-to-noise ratio is achieved by exciting a fluorochrome at the peak of its excitation curve and then detecting as much of the fluorescence as possible.
In any multiparameter flow cytometry application, the reagent panel consists of multiple fluorochromes that are carefully chosen to ensure they all have different excitation and fluorescence spectra. This is critical to enabling the instrument to separate the signals and thereby determine how much of each fluorochrome is attached to each cell. That, in turn, enables the instrument to unambiguously determine what type of cell it is.
The challenge is that both the excitation and fluorescence emission spectra are quite broad with long tails, making some crosstalk inevitable. There is also a natural variation in how much of a specific protein is expressed by each cell type. The task for the instrument designer and the engineer/scientist who designs the reagent panel is to minimize the crosstalk as well as the coefficient of variation (CV) in the final data.
The accepted approach (see Fig. 2) involves interleaving the excitation wavelengths and fluorescence detection windows. Signals in each detection window are plotted against each other to produce “scatter plots.” In research applications looking for both known and new cell types, the plots are often manually ringed. In clinical labs, the volume of testing makes this supervised analysis impractical, and multivariate computer analysis is instead used to automatically determine the identity of each cell.More wavelengths means enhanced performance
To maximize the number of parameters (proteins) and cell types that can be analyzed with minimum crosstalk, the excitation laser wavelengths are ideally spaced with fairly uniform separation. And the larger the spectral bandwidth over which the instrument can measure, the easier it is to accommodate more wavelengths while maintaining a large separation between each. Extending beyond the visible spectrum into the UV and near-IR is helpful in this regard.
However, this has all been historically complicated by available laser wavelengths. For example, the 488 nm wavelength (originally obtained from the argon ion laser) continues to be the de facto standard blue laser wavelength in flow cytometry, even though ion lasers are rarely used in this application. Similarly, the 532 and 561 nm wavelengths originally obtained from diode-pumped solid-state (DPSS) lasers continue to be the preferred green and yellow wavelengths.
There are two newer laser technologies that are scalable in both wavelength and power; this is important because cytometry instruments typically need 100–200 mW at each wavelength. These technologies are diode lasers and optically pumped semiconductor lasers (OPSLs).
By using both of these technologies, laser manufacturers now provide high-power sources at numerous new and legacy wavelengths across the visible and near-IR, filling in the gaps created by older laser types. For example, the 552 nm OPSL is now an alternative to the 561 nm wavelength in the yellow window, which could provide reduced crosstalk with the orange- and red-excited fluorochromes. And 458 nm diode lasers could eventually challenge those that are 488 nm as the blue standard because of reduced crosstalk with green-excited fluorochromes.
Thus, the scalability of both diode lasers and OPSLs has freed instrument designers to choose a panel of laser wavelengths solely based on overall instrument performance rather than the fixed wavelengths historically available. The inclusion of DPSS lasers in the designer’s arsenal has also enabled operation in the UV. An example is the 349 nm wavelength that optimally matches several newly available UV-excited fluorochromes.
From a practical standpoint, some manufacturers package their lasers in a common platform with a common interface, irrespective of the underlying laser technology. This simplifies the task of the instrument builder, as it allows the design of a single interface that can be used throughout the instrument, and even in different systems, without concern about which specific laser source type will ultimately be plugged into it.
Multiwavelength laser engines
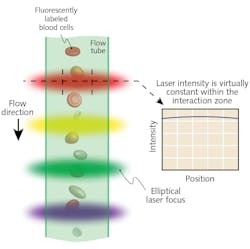
As the number of lasers in a single instrument increases, so does the beam alignment challenge. Each laser beam must be focused and shaped to a narrow ellipse at the flow stream. This ensures that even if cells are not in the center of the flow stream, they are still excited with the same amount of laser light. All of the ellipses have to be separately positioned in a series at the flow stream with micron spatial accuracy.
For a typical four-laser cytometer, this means four sets of focusing/shaping optics and four sets of beam delivery optics all must be perfectly aligned in a confined and congested instrument space. Moreover, the focusing/alignment requires high-stability optomechanics that won’t need frequent field maintenance. This all makes for a significant design and manufacturing challenge.
Coherent has massively reduced this challenge by developing a multiwavelength laser engine, called CellX, that delivers up to four independently addressable elliptical beams from OBIS lasers integrated inside the compact housing (see Fig. 3).During instrument assembly and testing, temporary removal of the cover provides separate precision adjustments for the beam pointing and focus of each beam. The cover is then replaced, leaving the optics locked securely in place to provide long-term alignment in the field. Thus, this integrated laser engine approach simplifies instrument design and assembly and speeds time to market. Additionally, it reduces system cost by eliminating redundancy in terms of power supplies and control electronics.
These laser engines are available with standard combinations of up to four of the wavelengths commonly used in most clinical assays: 405 nm, 488 nm, 561 nm, and 637 nm. They are also available populated with any of the 25-plus wavelengths from the OBIS series. Builders of research instruments are now sometimes combining two of these engines to get eight different visible wavelengths. They typically then add a standalone UV since these lasers are in a slightly large footprint at the requisite power level. All nine lasers are controlled through an identical interface, which simplifies the electronic and logistic design and manufacturing challenges, too.
High-dimensional cytometry provides the ability to measure more parameters at once, thus providing both researchers and clinicians valuable additional data. Laser manufacturers are supporting this trend in three ways: by packaging multiple laser technologies in a common format with a common interface to simplify the task of instrument design; by developing new wavelengths to improve instrument sensitivity and expand range; and—perhaps most important of all—by simplifying both system construction and service with integrated multiwavelength laser engines.
About the Author
Dan Callen
Product Line Manager, Direct-diode Laser Systems at Coherent
Dan Callen is Product Line Manager for Direct-diode Laser Systems at Coherent (Santa Clara, CA).
Matthias Schulze
Director of Marketing, OEM Components and Instrumentation at Coherent
Matthias Schulze is Director of Marketing, OEM Components and Instrumentation at Coherent (Santa Clara, CA).