Laser engines improve life sciences applications
Most photonics-based techniques successfully used in life sciences today are based on some form of fluorescence detection. These range from confocal microscopy used by researchers for live cell imaging, to flow cytometry that powers clinical blood counting instruments, to DNA sequencers, and more.
These applications often benefit from using multiple excitation wavelengths. In fluorescence microscopy, this allows users to excite a variety of targets, including traditional dyes, genetically expressed fluorescent proteins, and incipient cellular contents. In neuroscience, optogenetics techniques use one wavelength to simulate or silence target neurons, while a second wavelength maps the response in interconnected neurons via a fluorescent calcium indicator. The approach minimizes crosstalk between the activation and imaging channels. Flow cytometry often uses multiple wavelengths for multiparameter counting and/or sorting. This means using several wavelength-separated fluorochrome signals to analyze cells or other biological particles with a single instrument.
These disparate applications all share a common need to focus, shape, and position multiple laser beams with micron precision or better. Increasingly, this need is being met with “laser engines” that combine sources with highly stable free-space focusing optics or, alternately, integrated fiber delivery systems. For both OEMs and end users, this reduces costs, avoids time-consuming optical alignment procedures, shortens development times, and increases instrument stability and reliability.
Laser engines for multiparameter flow cytometry
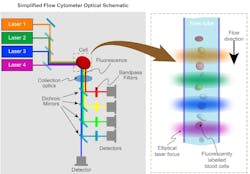
Laser engines were initially developed for flow cytometers, which are instruments routinely used in basic research to perform blood counts and to sort sperm or embryos for animal husbandry and fish farming. In flow cytometry, fluorescently labeled cells or other small targets are forced to flow in a single-file stream through a flow cell (see Fig. 1). They pass through an interaction zone of several laser beams that are shaped and focused into long lines arranged like rungs on a ladder. The resulting fluorescence and scatter are collected and separated into wavelength bands by dichroic and bandpass filters before detection. Some high-performance research instruments can have more than 100 separate detectors.
The first laser engines provided flow cytometry instrument builders with improved access to multiple laser wavelengths. However, these original laser engines were simply assembled from multiple individual lasers. This created inevitable redundancy, since each laser had its own controller, interface, and mechanical enclosure. In addition, they were built on a breadboard, so the user or instrument builder had to supply and align adjustable optics and mounts.
The second generation of laser engines was targeted squarely at OEM instrument builders. They feature miniaturization and use multiple laser cores. This translates into a single driver circuit board, interface, and power supply for all of the lasers, and it’s all contained within one compact enclosure.
In this type of laser engine, beam shaping and combining are included, and the focus and pointing of each laser are independently controlled by simple screw-type adjustments (one for each degree of freedom). The first of these products was offered with a choice of up to four of the most used wavelengths in flow cytometry instruments: 405 nm, 488 nm, and 640 nm, with an optional 561 nm (see Fig. 2).Today, builders of research instruments often incorporate two laser engines to access up to eight wavelengths from the ultraviolet (UV) up to 640 nm. This enables cell counting of up to tens of different data parameters with a single instrument.
Miniaturized optics, lower costs, superior stability
The use of flow cytometry continues to grow from research, to personalized medicine, to tracking the spread and progress of diseases like COVID-19. Market demand is also pushing instrument builders to shorten development times, reduce the size of benchtop models, lower their costs, and feature longer maintenance intervals.
Laser manufacturers are supporting this trend with a third generation of laser engines. They are based on miniaturized optics to achieve a smaller overall package with superior stability and greater economy.
Two key design innovations are required to achieve this reduction in package size. The first is simply to use smaller-diameter optics to begin with. This makes sense, since the internal laser beam diameters are less than 1 mm. So, there is no compelling reason to use the 0.5-in.-diameter lenses (~13 mm) commonly used in the past, along with their associated bulky mounts.
Second, the new engines do not use conventional optical mounts. These traditional mounts are assembled from multiple separate metal parts, flexures, and screws. In turn, they inevitably drift over long-term use due to thermal cycling and ambient vibrations.
The latest laser engines adapt a mounting technique originally developed for sealed intracavity laser use. It’s called PermaTrack, and its optics mounts are eliminated. Instead, the optics are aligned during manufacturing and permanently bonded to a stable substrate. With no separate mechanical components, there is no possibility for them to shift during normal use.
This mounting technology successfully eliminates one of the major reasons for field service in a flow cytometer: laser realignment benefiting both the instrument builder and the user. Moreover, since this latest laser engine technology is based on automated (i.e., robotic) assembly and alignment during production, it offers improved unit-to-unit consistency.
Eliminating the need to ever open the laser engine for realignment offers another substantial benefit. It allows the enclosure to be hermetically sealed so that the internal components are never exposed to any dust or chemicals that might outgas from organic materials. In addition, active “getters” are included to further protect a pristine environment. This approach is an adaptation of the same method that enables long-lifetime sealed visible and UV lasers for industrial applications.
In practice, factory-aligned and sealed laser engines are configured with the four (or three) beams in the usual arrangement of staggered focused lines. The relative separation of the lines has become relatively standardized throughout the industry, although OEMs can specify different geometries. The use of an adjustable focusing optic allows the position of the beam ladder to be adjusted in three dimensions to accommodate unit-to-unit variations in instrument assembly.
Plug-and-play systems for microscope users
This same design philosophy has also been applied to fluorescence microscopy, a technique that can be found in just about every life sciences research lab—from universities to pharmaceutical manufacturers. Integrated solutions for introducing multiple laser wavelengths into these microscopes have been available for some time.
Most commonly, this is accomplished via fiber coupling. This avoids cluttering the immediate microscope space with optics and associated hardware. But it also means that multiple lasers must be aligned into fibers and then all coupled together into a single input fiber to the microscope.
For fluorescence microscopy, single-mode fiber is usually preferred, because this results in higher efficiency and cleanly focused (higher-resolution) sample excitation. But a typical single-mode optical fiber has a core diameter in the 8.0–10.5 µm range. It is notoriously time-consuming to align a laser beam into this size of fiber core using a conventional fiber-coupling mount with five or six degrees of freedom. Even an experienced technician might need several hours to complete this task for each laser.
Once alignment is achieved, there is the problem of maintaining the alignment at the necessary micron-scale during use and over time. Handling the instrument and normal variations in ambient temperatures can easily put it out of alignment. Plus, there is a challenge of using dichroic filters, polarizers, and waveplates to combine the wavelengths.
Laser manufacturers have addressed this problem with compact modules that act just like an optical bus. These modules combine the output of multiple fiber-coupled laser sources into one single-mode fiber that feeds the microscope. The user provides input through eight wavelength-specific fiber ports, which use snap-in connectors. The output is a single optical fiber with a constant numerical aperture (NA) over the entire 405–640 nm range, and no user adjustments are necessary.
Compact and smart solid-state lasers today are nearly as simple to operate as a lightbulb. This has enabled life science researchers and instrument builders to focus on their work, rather than on laser adjustment. This, in turn, has led to the proliferation of fluorescence-based methods in laboratory and clinical settings. The ongoing evolution of laser engines that provide this same easy access to multiple wavelengths will continue to give fluorescence techniques a bright future.
About the Author
John Abbott
Director of LMC Sales, Coherent
John Abbott is Director of LMC Sales at Coherent (Santa Clara, CA).
Matthias Schulze
Director of Marketing, OEM Components and Instrumentation at Coherent
Matthias Schulze is Director of Marketing, OEM Components and Instrumentation at Coherent (Santa Clara, CA).
Kim Netzeband
Director, Life Sciences Marketing at II-VI Incorporated
Kim Netzeband is director of life sciences marketing at II-VI Incorporated (Saxonburg, PA).