Optogenetics: Ten years after - Optogenetics progresses in clinical trials
Work in optogenetics (one of Scientific American's "Top 10 Emerging Technologies of 2016" [see http://bit.ly/2bCNylr]) has entered a new era. Not only did the technique recently celebrate a decade of progress using the light-sensitive protein channelrhodopsin-2 (ChR-2) in mammalian neurons,1 but in August 2016, RetroSense Therapeutics (Ann Arbor, MI) announced success in its initial clinical trial (see http://bit.ly/2b6t8jf)—the first to involve therapeutic application of optogenetics.
The trial, begun in February 2016, is part of a study to test dose levels of RST-001, a therapy that delivers ChR-2, to cells in the retina of the eye. When expressed and exposed to light including blue wavelengths, the ChR-2 protein can produce an electrical signal that is carried to the brain. The idea is to replace the function of rod and cone photoreceptors damaged by disease.
The U.S. Food and Drug Administration (FDA) cleared RetroSense's Investigational New Drug (IND) application in late 2015. The trial began when a woman with advanced retinitis pigmentosa (a degenerative condition that destroys light-sensitive cells in the retina and leads to severe vision loss)—one of a cohort of study participants—received injections aiming to impart light sensitivity to the eye's ganglion cells. The goal of the therapy is to restore at least some vision-enough to help an affected person become more independent. The goal of the clinical trial, which will continue for two years, is to evaluate the safety of RST-001.
Augmented gene therapy
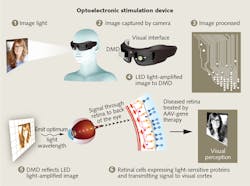
Meanwhile, GenSight Biologics (Paris, France), a clinical-stage biopharmaceutical company, is working toward clinical trials with its own method to address retinitis pigmentosa. The company presented progress in animal studies of its product candidate at the 2016 Association for Research in Vision and Ophthalmology annual meeting (April 30-May 5). GenSight's gene therapy uses an opsin activatable with red light, the ChrimsonR-tdTomato (ChrR-tdT), which was developed by optogenetics pioneer Ed Boyden of the Massachusetts Institute of Technology (MIT; Cambridge, MA). "The natural range of light sensitivity of human photoreceptor cells is larger than that of channelrhodopsins," the company notes, so to deliver light at an intensity and wavelength to effectively stimulate the altered retinal cells, GenSight's approach uses accessory hardware. The GS030 comprises:
- Goggles that include an asynchronous time-based image sensor (ATIS, or "neuromorphic video camera") and a microprocessor-driven digital micromirror array that carries visual information signals and light to the macula, the central portion of the retina, which provides the clearest vision; and
- A cellphone-sized computer connected to an optoelectronic stimulation device, which processes visual information and controls a light source to encode and amplify light signals at a specific wavelength.
Antonello Bonci, scientific director of the intramural research program at the National Institute on Drug Abuse (Rockville, MD), says the clinical work will produce "a gold mine of information about doing optogenetics studies in humans."
Addiction and substance abuse are just some of the disorders that optogenetics has enabled researchers to study more effectively. To help others design behavior experiments that can be readily interpreted, scientists at MIT have published a guide.2
Among the areas in which the technique has facilitated research are sleep, hearing, smell/odor detection, attention, and locomotion. Scientists are working on studies to address recovery for an impressively broad range of conditions: Parkinson's disease, stroke, seizure disorders, schizophrenia, depression, anxiety, obsessive-compulsive disorder, autism, diabetes, obesity, cancer, and cardiovascular disease, and to provide pain relief.
Pain relief may well be the next therapeutic application to reach clinical trial. Circuit Therapeutics (Menlo Park, CA), a company cofounded by optogenetics pioneer Karl Deisseroth of Stanford University (Palo Alto, CA), has demonstrated the ability to block neural circuits with yellow light. The approach reports to overcome limitations and side effects of electrical stimulation (the current standard of care for the treatment of epilepsy and Parkinson's disease), for instance, and the company has presented preliminary data at the 2016 American Society of Gene and Cell Therapy (May 4-7, Washington, DC).3
Photonics technologies
Lasers, laser diodes, LEDs, optical fiber, cameras, lenses, mirrors, beam modulators, and other optics and photonics components are critical to optogenetics, of course. While the impact of research progress on these technologies has not been quantified, new market research by Credence Research estimates the impact of optogenetics research on providers of sensors (fluorescent proteins, chloride sensors, etc. for monitoring neural circuits), actuators/effectors (various opsins for directly manipulating such circuits), and targeting techniques (transgenic animals, etc.). The report ("Optogenetics: Market Growth, Future Prospects and Competitive Analysis, 2016-2022; see http://bit.ly/2b6tYfD) estimates a CAGR of 18.6% for the next six years, forecasting that these segments will be worth $57.2 million by 2022 (2015 market value was $17.2 million).
REFERENCES
1. K. Deisseroth, Nature Neurosci., 18, 1213–1225 (2015).
2. B. D. Allen et al., Learn. Memory, 22, 4, 232–238 (2015); doi:10.1101/lm.038026.114.
3. S. Reardon, Nature, doi:10.1038/nature.2016.19886 (2016).
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.