LASER SAFETY: Retinal injury research impacts future laser safety standards
KARL SCHULMEISTER
When the eye is exposed to laser radiation with sufficient power, permanent injury results. Since the invention of the laser, a number of research groups—most notably the US Army and Air Force—conduct bioeffects research to determine injury thresholds for available wavelengths and pulse durations. The collective dataset of laser injury thresholds forms the basis to define safe exposure levels, as given in the American National Standards Institute (Washington, DC) ANSI Z136 standards series in the United States and by the International Commission on Non-Ionizing Radiation Protection (ICNIRP; Oberschleissheim, Germany) and the International Electrotechnical Commission (Geneva, Switzerland) IEC 60825 series on the international level.
The task of setting safe laser exposure limits is a challenging one, as the injury thresholds vary strongly with pulse duration and wavelength, and have different underlying mechanisms of interaction such as thermal, or for sufficient photon energy, photochemical. The threshold for thermally induced injury also depends on the diameter of the laser beam that is incident on the tissue. Further dependencies arise for multiple pulses where thresholds vary with repetition rate and number of pulses.
For injury of the retina, which is the most critical one, the imaging properties of the eye result in experimental challenges—for instance, in the determination of retinal image diameter—and also require that non-human primates (NHPs) such as rhesus monkeys are used as the primary model.
Even though injury threshold research has been generating data for more than 40 years, the dataset is not complete for all parameter regimes due to the large parameter space and multidimensional dependencies. Considering the cost for NHP experiments and that the default condition for laser beams is to produce a minimal retinal spot size, it is not surprising that there is little NHP data for extended retinal images.
To complement available NHP data, the laser group at Seibersdorf Laboratories (Seibersdorf, Austria) developed an ex vivo cow eye model as well as a computer model that produces a large array of threshold data for different spot sizes and pulse durations, and is also a tool to analyze the risk for injury with specific products. A threshold study for the 532 nm wavelength was published in 2008 and recently complemented with a dataset in the near-infrared (NIR) 1090 nm region.1,2 The data form the basis of an ongoing effort to update laser maximum permissible exposure (MPE) values in terms of spot-size dependence that will allow laser power levels a factor of up to 20 times higher than for current limits.3
New spot-size data
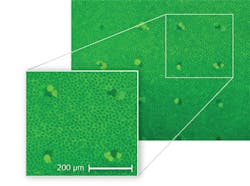
In preparation for recent retinal exposure experiments at Seibersdorf Laboratories, ex vivo samples were obtained from fresh cow eyes in which the retinal pigment epithelial (RPE) cells were kept alive for several hours. The cell viability after exposure was determined under a microscope with fluorescent staining, where dead cells appear dark and living cells fluoresce (see Fig. 1). Using a computer-controlled laser exposure system, the cow-eye model was validated against rhesus monkey data for a 100 ms pulse duration and found to be in good agreement.4 A computer model was also developed that could predict injury thresholds both for the rhesus monkey as well as for the cow-eye model with good reliability.
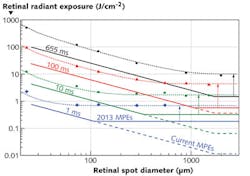
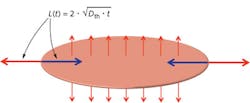
When the threshold data are plotted as a function of retinal spot size in terms of retinal radiant exposure (equivalent to radiance dose), two regions can be distinguished to the left and right of a critical spot size: On the small spot side, the thresholds decrease linearly, while to the right of the critical spot size, the thresholds no longer depend on the spot size (see Fig. 2). In principle this basic trend was known before and also reflected in the MPEs in the correction factor CE. What was not known is how the trends vary for different pulse durations: The critical spot size shifts to smaller spots for shorter pulse durations.With the help of the computer model it is possible to study and understand the critical role of radial heat flow. The difference between the two regimes is whether or not radial cooling affects the temperature at the center of the spot at time periods shorter than the pulse duration. The RPE, a single-cell layer in the retina, forms a very thin (on the order of 5 μm) highly absorbing layer, so that the incident laser beam, assumed to have a top-hat profile for simplicity, produces a heat source in the shape of a thin disk with a certain diameter D. Heat flows outward into non-irradiated areas and thus the edge of the disk is cooled.
One can think of the effect of the radial outward heat flow as a "cooling wave" that flows inward toward the center of the laser spot (see Fig. 3). As long as that cooling wave does not reach the center of the spot, this center is surrounded by areas of the same temperature and the overall temperature continues to increase with ongoing laser exposure. From thermal diffusion theory it is known that for a given time t, a heat wave (or cooling wave) travels through a medium over a distance L = 2√(Dth t) where Dth is the thermal diffusivity; for water Dth = 0.14 mm2/s.When the cooling wave reaches the center of the spot during the pulse, the center does not reach as high a temperature as it did when it was not affected by radial heat flow. The smaller the spot, the sooner the cooling wave reaches the center, and the lower the temperature will be for a given retinal irradiance, which produces the 1/D dependence of the injury threshold on the left side of the critical spot size. To the right of the critical spot size, the spot is so large that the center is not cooled radially during the pulse—the center does not thermally "see" any edge effects—and for that condition, the central temperature is independent of the spot size, and so is the injury threshold.
The variation of the critical spot size with pulse duration comes about because the shorter the pulse duration, the smaller the spot must be so that radial heat flow can have an effect on the center of the irradiated spot. The experimentally observed critical spot radius for different pulse durations is practically equal to the thermal diffusion length for that pulse duration, showing how well the observed trend can be explained by radial heat flow effects.
Pulse-duration- dependent αmax
Retinal image size is characterized in laser safety standards by the angular subtense of the image, which is equal to the angular subtense of the source and is given the symbolα.5 The critical spot size beyond which the MPEs are not spot-size dependent is given the symbol αmax. In the current MPEs,αmax has a constant value of 100 mrad (equivalent to 1.7 mm in the human eye), which we found to be applicable only for pulse durations of about 250 ms. For shorter pulse durations, the current constant value of 100 mrad leads to MPEs that continue with the 1/α (when expressed as retinal radiant exposure, or radiance dose) or asα-dependence (when expressed as corneal exposure), while the injury thresholds are correspondingly higher for spot sizes beyond the critical spot diameter.
By introducing a pulse-duration-dependent αmax that represents the experimentally found values of the critical spot size, the MPEs can be raised while still assuring an appropriate reduction (safety) factor between injury thresholds and MPEs. The formula for αmax in units of mrad equals 200 √t (t in seconds limited to the range of 625 μs to 250 ms), so that for pulse durations less than 625 μs, αmax = 5 mrad and for pulse durations above 250 ms, αmax = 100 mrad. Thus, in terms of spot-size dependence, the MPEs will not change for continuous-wave lasers where αmax will still be 100 mrad, and there will be no change—regarding the spot-size dependence—for pulsed sources that produce minimal retinal image (such as for well-collimated beams). However, the MPE levels for pulsed sources that form an extended image (such as some fiber sources or line lasers at close range) are increased by up to a factor of 20.
Lower MPEs in the nanosecond regime
In recent years, ex vivo models were also used at the Wellman Center for Photomedicine (Boston, MA) with bovine eyes and at the Medical Laser Center Lübeck (Lübeck, Germany) with porcine eyes to study the formation of microbubbles around the highly absorbing pigment particles (the melanosomes) in the RPE.6,7
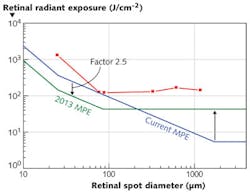
When the micrometer-sized melanosomes are heated by a laser pulse at time durations before the heat flow starts to have an effect, they are "superheated" compared to a homogeneously absorbing medium with the consequence that vapor bubbles can form around the melanosomes and cause cell death. But for pulse durations in the nanosecond regime, microbubble formation occurs at radiant exposure levels lower than are necessary to induce thermal "bulk" injury of the cell. This phenomenon, together with a more restrictive endpoint for the observation of the threshold in recent NHP studies, explains why, for 5 ns pulses, lower injury thresholds were found as previously seen, leading to the necessity to lower the MPE in the nanosecond-pulse-duration regime.8The reduction of the MPEs by a factor of 2.5 shifts the break point in the pulse-duration dependence from 18 μs to 5 μs. Together with the new spot-size dependence, the future MPEs follow the injury thresholds more closely, with a more homogeneous reduction factor (see Fig. 4). The larger reduction factor of about 10 for the minimum spot regime is needed to account for the uncertainty regarding the actual spot size at the retina for an exposure to a well-collimated beam. It should be noted that it is also planned to amend the treatment of multiple pulses, so that for most pulsed systems, the overall effect will be an increase of permitted energy per pulse, even though the MPE for a single pulse is reduced.
REFERENCES
1. K. Schulmeister et al., J. Biomed. Opt., 13, 054038 (2008).
2. K. Schulmeister et al., "Near infrared ex vivo bovine and computer model thresholds for laser-induced retinal damage," Photonics and Lasers in Medicine, accepted March 2012.
3. K. Schulmeister et al., Health Physics, 100, 2, 210–220 (2011).
4. D.J. Lund et al., J. Biomed. Opt., 12, 024023 (2007).
5. R. Henderson and K. Schulmeister, Laser Safety, New York, London: Taylor & Francis Group (2004).
6. H. Lee et al., J. Biomed. Opt., 12, 064034 (2007).
7. G. Schuele et al., Investigative Ophthalmology & Visual Science, 46, 2, 714–719 (2005).
8. J.A. Zuclich et al., J. Laser Appl., 12, 74–80 (2000).
Karl Schulmeister is a member of ANSI, ICNIRP, and IEC laser safety committees and is a laser product safety consultant at Seibersdorf Laboratories, 2444 Seibersdorf, Austria; e-mail: [email protected]; www.seibersdorf-laboratories.at/en.