ULTRAFAST SOURCES: Ultrafast lasers produce nanoparticles
BING LIU, ZHENDONG HU, AND YONG CHE
Ultrafast-pulsed lasers are used in many industrial applications that utilize the process of ultrafast-pulsed-laser ablation, including laser machining, medical surgery, and chemical analysis. Although the pulse energy used in practice varies widely from the microjoule to the millijoule level, tightly focused microjoule pulses are intense enough to reach above the ablation threshold of most materials. Higher-energy pulses become less efficient by inducing more ionization and heat, which can cause more damage to the target. For these reasons, ultrafast fiber lasers-with their high repetition rate (up to megahertz levels) and moderate microjoule pulses-are especially suitable for applications that require high accuracy and high processing rate.
Materials synthesis using pulsed-laser deposition (PLD) is another important application being explored for ultrafast-pulsed lasers. While high-power nanosecond pulsed lasers (excimer and Q-switched Nd:YAG) are traditionally the workhorses for material synthesis, ultrafast lasers offer higher material removal efficiency and higher deposition rates than nanosecond lasers, which have high pulse energy of hundreds of millijoules and low repetition rates of less than 100 Hz.
Automatic particle generation
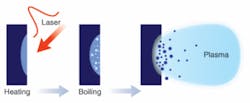
An interesting byproduct of the process of ultrafast PLD is the automatic generation of nanometer-scale particles during ablation.1, 2 The mechanism of this automatic nanoparticle formation is generally believed to be related to the phase transition process under extreme conditions of high temperature and high pressure (the so-called critical conditions) produced by the ultrafast laser pulses (see Fig. 1). The ultrashort femtosecond pulse duration is only long enough for the free electrons in the material to heat up. The thermodynamic characteristics of the irradiated material will keep evolving after the pulse ends. Because the time is still too short for significant heat conditions to occur, the ensemble of electrons and lattice atoms will reach a transient high temperature that can be higher than the critical point of the material, and the pool of melt will experience violent phase transitions-such as boiling-and expand its volume. If the speed of expansion of the melt exceeds the yield of the surrounding solids, the buildup of high pressure can cause material removal to occur in an explosive fashion, resulting in a mixture of ionized vapor and a large number of nanoscale droplets.
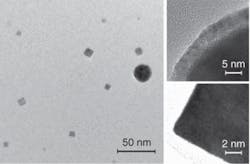
In view of the many potential applications of nanoparticles and the ongoing research into ways to produce them, it is worthwhile to evaluate ultrafast-pulsed-laser ablation as a method of producing nanoparticles. During ablation of nickel (Ni), for example, a small amount of oxygen is provided in the background to explore the possibility of obtaining nickel oxide (NiO), which is an important magnetic material (see Fig. 2). The ablation process produces two types of particles that differ in size and shape: the larger ones are round and have an oxide shell, but the small ones have a cubic shape and are very well crystallized. Further analysis has verified that the small cubes are indeed NiO, and the large particles have a shell/core structure made of NiO/Ni and have interesting magnetic properties due to the magnetic interaction between the core and the shell.The large round particles, which may be undesirable, result more often at high laser fluences when using high pulse energies. It appears that, because of the Gaussian beam profile, the center of the laser focal spot experiences a higher temperature and a higher pressure than the edge of the focal spot, causing “splashing” in the melts, which results in the large particles. The use of moderate to low pulse energy would eliminate the large particles and achieve a narrower size distribution.3
Alloy nanoparticles
Ablation of alloy targets has also provided encouraging results. Alloy nanoparticles are of interest because of the possibility of tuning their physical and chemical properties by varying their alloy compositions, a challenging task for alternative fabrication methods. Using ultrafast-fiber-laser ablation, nickel-iron (NiFe) alloy particles can be obtained, for example (see Fig. 3).4 The nominal nickel content of the alloy target is 80% (which makes the so-called permalloy for its high magnetic permeability and low magnetic coecivity). The chemical composition of alloy nanoparticles can be determined by energy-dispersion spectroscopy (EDS) of the elements’ characteristic x-ray emission induced by a high-energy transmission-electron-microscopy (TEM) electron beam. It is encouraging to see that all the measured particles have the same desired Ni and Fe composition of 80% and 20%, respectively, regardless of their size.The preservation of the alloy composition ratio may be related to the properties of the NiFe alloy itself: it is known that nickel and iron can make homogenous liquid solutions for a wide range of compositions. Therefore, when the alloy target is quickly melted during the early stage of ultrafast-laser ablation, the pool of melt remains a chemically homogeneous liquid. Also, the timescale of ultrafast-pulsed-laser ablation can be too short for any significant phase separation to occur.
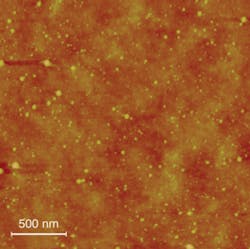
Another apparent but remarkable advantage of using ultrafast-pulsed-laser ablation for nanoparticle generation-compared to other processes such as wet chemical methods-is that laser ablation is nonselective regarding the target material and is therefore especially applicable to precious, inert, and high-temperature metals. Furthermore, because the particles are formed during ablation, the substrates for collecting the particles can be maintained at room temperature, allowing heat-sensitive substrates such as glasses and polymers to be used (see Fig. 4).Ultrafast-pulsed-laser ablation can be used to generate crystalline and polycrystalline nanoparticles with various morphological qualities, such as nanocubes and shell/core structures. Alloy nanoparticles have also been successfully fabricated with well-preserved and controllable alloy compositions. These demonstrations make ultrafast fiber lasers with their high average power at a suitable pulse energy and high repetition rates excellent laser sources to produce large quantities of nanoparticles.
REFERENCES
1. S. Eliezer et al., Phys. Rev. B69, 144119 (2004).
2. S. Amoruso et al., Phys. Rev. B71, 033406 (2005).
3. B. Liu et al., Appl. Phys. Lett.90, 044103 (2007).
4. B. Liu et al., Proc. SPIE6460, 646014 (2007).
BING LIU and ZHENDONG HU are members of technical staff and YONG CHE is manager of new functional material research at IMRA America, 1044 Woodridge Ave., Ann Arbor, MI 48105-9774; e-mail: [email protected]; www.imra.com.