Early clinical studies of a prototype carbon dioxide (CO2) laser being developed by ESC Medical Systems (Yokneam, Israel) have demonstrated that the laser's unique wavelength—9.6 µm—may prove more effective for treating hard dental tissue than the erbium-based lasers that already have US Food and Drug Administration (FDA) approval for this application. The system has been in development since 1998, and the company is now preparing to launch a commercial version in Europe and to begin full clinical trials in the USA.
The 9.6-µm wavelength offers several unique properties that make it particularly appropriate for treating hard dental tissue, according to Dr. Harvey Wigdor (Ravenswood Hospital Medical Center; Chicago, IL), who spearheaded the initial research and evaluation of the ESC laser. In particular, its high absorption coefficient in both water and hydroxyapatite (the predominant crystal structure in dentin and enamel) make it a very efficient cutting tool. At the same time, the laser's energy has little effect on surrounding pulpal tissue and the internal structure of the tooth, making it very attractive for a variety of dental applications.
"Three to four years ago, a number of studies showed that the 9.3-9.6-µm wavelength range has a very high affinity for hydroxyapatite," Wigdor says. "In addition, because the water absorption of the 9.6-µm wavelength is very similar to that of the 10.6-µm wavelength, this could actually be both a hard- and soft-tissue laser."
Prior to working with the 9.6-µm CO2 laser, ESC was already developing an erbium laser for dentistry. While the erbium wavelengths are well absorbed by enamel, they do not have the same affinity for hydroxyapatite as the CO2 laser. Thus, although ESC now has an erbium laser and a diode laser on the market for dentistry, the company decided to explore the 9.6-µm laser using technology originally developed and patented by Laser Industries (now an ESC subsidiary).
The company went to the FDA last year and gained approval to do a pre-investigational-device-exemption (IDE) study of the 9.6-µm laser on human teeth. The pre-IDE study was conducted by Wigdor and his colleagues at Northwestern University (Evanston, IL) and the University of Illinois College of Dentistry (Chicago). Results were presented at the SPIE Photonics West/Biomedical Optics meeting last January in San Jose, CA.
No anesthesia needed
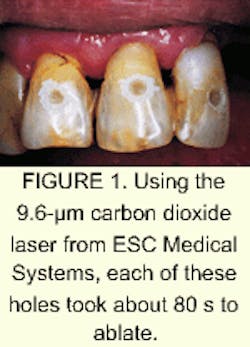
The laser emits 50-mJ, 60-µs-long pulses of laser energy with a 300-µm diameter spot size. The dental handpiece includes a scanner that produces a 2-mm beam that is then focused to the 300-µm spot.A total of 24 teeth were treated during the study. All teeth were treated in the mouth, but each tooth had already been scheduled for extraction, so the effects of the laser were studied after the teeth were removed. The number of teeth treated per patient varied from two to four.
Each tooth was treated with 2.5-3.5 W of energy to tissue. Continuous water spray was used as a cooling mechanism. No anesthesia was applied prior to lasing, and Wigdor says the patients experienced no discomfort.
As part of the study, the researchers compared the average time for cutting the holes with a laser to the time it takes to create a similar hole with a conventional dental handpiece. They found that, for cutting a hole in enamel and dentin that were equal in size, a conventional handpiece averaged about 50 s per cut, while the 9.6-µm CO2 laser averaged about 80 s (see Fig. 1). This means the laser is comparable to existing nonlaser technology in terms of surgical efficiency, which could be an important selling point for ESC once the laser becomes commercially available.
In addition, Wigdor reports that the ESC laser cuts better than any laser he has ever used and that the effect of the laser energy on surrounding dental pulpal tissue was minimal (see Fig. 2). Again, when comparing the effects of the laser to those of a conventional handpiece, the researchers found that the pulp in all of the holes appeared similar and had no apparent inflammation or vascular changes.
Floor-size prototypes of the laser are now in use at sites in Europe and Israel, according to Yacha Sutton, CEO of ESC. The company reportedly is gearing up for a major commercial push of this laser in Europe, but Sutton says the system is still undergoing clinical investigation in the USA and internationally.
"We have not pushed this product too much, but we are looking at it and reconsidering it," he says. "We are in the preliminary stages of putting together a full clinical trial in the USA."
Kathy Kincade | Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.
