Chalcogenide glasses are the basis for emerging applications in infrared technology
GERNOT WEBER
For decades, chalcogenide infrared (IR) glasses (chalcogenides) were considered “exotics.” For the rather-small number of applications that relied on IR wavelengths, other IR materials performed well. As a result, demand for chalcogenides was quite limited. Low demand for the material restricted producers to small businesses that had a hard time providing detailed technical descriptions of their products or reproducible, consistent results. The result was a self-fulfilling prophecy: low demand meant low availability of material, and thus a reluctance on the part of optical designers and product researchers to experiment or explore chalcogenides as a material solution to new products.
An emerging set of potential applications, along with increased interest in IR designs that are smaller, lighter, and do not require relatively expensive internal cooling systems, has created a new interest in chalcogenide glasses. In turn, there is new demand, increased availability of high-quality materials, and more-thorough technical descriptions of chalcogenide glasses (see Fig. 1).
Matching the wavelength to the application
For the purpose of developing applications, the IR spectrum is commonly divided into three spectral bands: shortwave (SWIR), medium-wave (MWIR), and longwave infrared (LWIR). There are advantages and disadvantages to each band that make some applications more suitable than others.
SWIR (about 1 to 3 µm in wavelength) tends to produce the clearest images, because as with visible light, photons are reflected, absorbed, and scattered by objects, creating the high contrast needed for strong resolution. The sensors that process SWIR tend to be very expensive. Because of these tradeoffs, SWIR-based applications typically concentrate in high-value areas, such as anticounterfeiting, process quality control, medical imaging, and semiconductor manufacturing.
MWIR applications (about 3 to 5 µm) are ideal for detecting sudden temperature fluctuations and avoiding scattering from atmospheric conditions. MWIR systems tend to require internal cooling, making them more expensive. MWIR systems are something of a rarity, typically used in mission-critical applications such as defense (identifying the exhaust of fighter jets) and industrial thermography.
LWIR (about 8 to 14 µm) has the broadest range of applications, particularly in the civil security sector. LWIR cameras are mainly used to measure surface temperature profiles of objects with high accuracy; such systems make heat radiation visible even at great distances or in total darkness. Image resolution on LWIR can be quite poor, but this tends not to disqualify LWIR for applications that include building inspection, thermal imaging surveillance, personal night vision, search and rescue tools for firefighters, and other low-cost uncooled systems.
In years past, the core use of most IR systems has been confined to military and industrial sectors. But there’s been a general trend toward the use of IR cameras in other sectors. SCHOTT (Mainz, Germany) is seeing increased potential for civil-security equipment, commodity-grade consumer electronics, and in the noncontact IR thermometers that organizations and businesses are using to guard against COVID-19 (see Fig. 2).But because of the expense of cooling systems and processors associated with SWIR and MWIR, it’s quite likely that designers will focus on LWIR systems. Fortunately, LWIR applications lend themselves well to optical designs that are less complex and materials that can be manufactured in large quantities. Furthermore, these materials can be combined with each other to achieve optimal imaging performance.
Matching the material to the application
A second consideration in the design of IR cameras is the material that will be used in the lens system. For IR imaging systems, whether they are night-vision devices, thermal imaging cameras, motion control systems, pyrometers, or diagnostic equipment, the optical materials used in the systems must meet very special requirements.
Many glass materials, including common formulations made from soda-lime or specialty glasses used in other industries, are opaque to LWIR and MWIR. Therefore, they are not suitable for applications using these applications. This opacity is caused by the absorption of IR radiation by molecular vibrations of the glass matrix, which is related to the silicon-oxygen bond. Chalcogenide glasses replace silicon with metals or semi-metals like arsenic, germanium, antimony, or gallium; oxygen is replaced with sulfur, selenium, or tellurium (these are the chalcogens on the periodic table). In fabricating chalcogenide glasses, the semi-metal is mixed with the chalcogen. The result is an amorphous structure that can be molded and pressed into final shape.
The ability to mold and shape chalcogenide glasses is a significant material property that contrasts with more common IR materials, such as germanium, zinc sulfide, zinc selenide, and silicon-based materials. These materials have crystalline structures and can perform quite well at SWIR, MWIR, and LWIR wavelengths, depending on the application. These materials must either be produced in a form that is near the shape the end user requires or they need to be ground and polished into their final form, a time-consuming process not amenable to mass-market production. Single-point diamond turning is another approach to optical fabrication of many of these materials.
These IR materials do have some advantages. For example, germanium lenses tend to be a preferred substrate for diamond-like coatings; in addition, its high refractive index tends to lend itself to shorter optical paths. Zinc sulfide has a very broad range of transmission, from the visible light spectrum to about 19 µm.
Due to constant development in the area of uncooled IR detectors, a consistent demand exists for materials with well-defined thermal properties.
One thermal property is the temperature coefficient of refractive index (dn/dT). This property describes the degree to which the refractive index of the material changes in response to heat in the relevant temperature ranges. Systems that rely on materials with poor dn/dT need to compensate in other ways (and thus increase complexity) to avoid degradation of the image during temperature swings. Germanium and silicon lenses have particularly poor dn/dT. Chalcogenides, on the other hand, offer much better performance on this front.
A second thermal property is the coefficient of thermal expansion (CTE), which describes the degree to which a material expands or contracts in response to temperature changes. IR glasses have a CTE that matches that of aluminum, making it much easier to, for example, mount a lens in an aluminum ring. For sake of comparison, the CTE for germanium in the relevant temperature range is 5.8 × 10-6/K. For aluminum, it is 23.1 × 10-6/K; for IRG 26 glass, it is 21.4 × 10-6/K.
Traditionally, crystals such as zinc sulfide and zinc selenide did not lend themselves to color-corrected systems due to the material’s difficult-to-work-with dispersive properties. But with chalcogenide glasses, color-corrected systems containing these crystals are now possible.
New requirements for imaging optics
Consistent innovation in the IR camera industry has paved the way for new applications. A constant search for materials that offer high performance at prices the consumer-electronics industry finds palatable has meant that the requirements for imaging optics have inevitably changed. This relates not only to technical and physical properties, but also to the costs and processing of the IR materials.
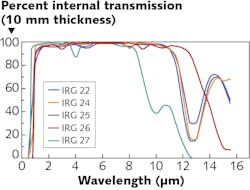
Properties such as a broad transmission spectrum over the wavelength range (see Fig. 3), stable transmission at different temperatures, constant dispersion over the IR wavelengths, and a low dn/dT are technical requirements for product development that many optical designers did not have access to. In response, companies have begun investing in the development of materials data that engineers and optical designers can evaluate for product development.However, in order to be able to provide inexpensive IR optics to volume markets, it is necessary to switch to different manufacturing processes in the area of component manufacturing. In contrast with crystalline optical materials, chalcogenide glasses have material properties that boost economical processability, enabling cost-effective volume production of components.
As a result, IR glasses can make a major contribution to enabling innovative solutions in optical systems to serve the rapidly growing segment of infrared technology.
Gernot Weber is Worldwide Product Manager IR Glass at SCHOTT, Mainz, Germany; e-mail: [email protected]; www.schott.com.