BILL RIEGLER, ROB THOMAIER, AND STEVE BRUNER
Silicone’s polymer chemistry and structure provide different types of silicones in a broad spectrum of material compositions that make silicone a viable option for numerous high-brightness (HB) LED packaging applications, such as die encapsulation, die-attach adhesive, and lens-type material. Modifying the polymer chemistry can produce a silicone with a refractive index ranging from 1.38 to 1.60.
A Lumileds (San Jose, CA) patent filed in 2002 discusses the problem of a large-particle-size phosphor (2 to 20 µm)—embedded in a lower-refractive-index (1.50) “host” material, like silicone—causing light losses due to scattering. As more phosphor was added, the problem became worse. The problem continued to worsen when the phosphor’s refractive index was increased without increasing the refractive index of the host material.
Lumileds provided a statistic of 50% light power lost due to this scattering effect. The patent suggested several ways to overcome the problem, including decreasing the phosphor’s particle size, increasing the refractive index of the host material, and improving the dispersion of the phosphor into the host material.
Material composition
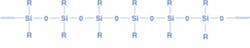
Silicones are inorganic polymers, formed of repeating oxygen and silicon atoms with no carbon atoms in the backbone (see Fig. 1). Different groups can be added to the polymer backbone, imparting characteristics like high refractive index or solvent resistance. Different forms of the same material can be produced, namely gels and thermosets for device packaging.
Silicone gels contain reactive silicone polymers and reactive silicone crosslinkers in a two-part system. Mixed together, these materials have a soft, compliant feel when cured, and stick to substrates without migrating. Viscosities can be adjusted with the molecular weight of the polymers from 200 to 10,000 centipoise (cP). Depending on the polymer’s functionality, optical-index matching can be achieved from 1.38 to 1.57. For HB LED applications, this capability allows the optimal amount of light to come out of the die, while protecting it from dust, moisture, vibration, and temperature changes. The gel’s yield strength is low enough to permit wire bonds to slice through during thermally induced micromotion without risking wire-bond failure. Other applications, besides encapsulating HB LEDs, include potting of packaged modules such as transponders, transceivers, and detector arrays.
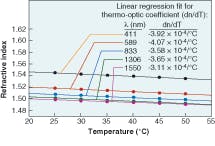
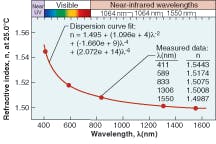
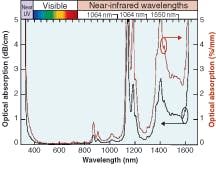
Silicone thermosets fall into two categories: moldable elastomers and adhesives. Like the gels, these two-part systems contain reactive polymers and crosslinkers that cure to a rubbery hardness. Most will cure at room temperature; however, some need heat to cure. To impart improved physical properties, these materials typically have higher viscosities. The moldable materials can be cast or injection-molded into HB LED lenses. They have inherently stronger physical properties than gels and can work as excellent adhesives in optical applications. These materials also can have the broad refractive-index range of 1.38 to 1.57.Refractive index is based on the measurement of the speed of light traveling through a transparent material. It is measured at 589 nm (the sodium D line, or “nD”), with a refractometer using the method of ASTM D-1218 (a standard test method for refractive index and refractive dispersion of hydrocarbon liquids) at a fixed temperature of 25°C (see Table 1). The refractive index of a silicone material also varies with temperature and wavelength (see Fig. 2 and 3).
| Material | Acronym | Trade names | nD |
|---|---|---|---|
| Water | 1.34 | ||
| Polytetrafluoroethylene | PTFE | Teflon | 1.34 |
| Magnesium fluoride | MgF2 | 1.38 | |
| Fused quartz | 1.46 | ||
| Acrylate | PMMA | Plexiglass | 1.49 |
| Cyclic olefin | COC | Topas | 1.53 |
| Diphenyldimethylpolysiloxanes | PVMQ | Up to 1.60 | |
| Polycarbonate | PC | Lexan | 1.59 |
| Aluminum oxide | Sapphire, ruby | 1.76 | |
| Yttrium aluminum garnet | YAG | 1.8 | |
| Galium nitride | GaN | 2.5 to 2.7 | |
| AlGaInP | 3.25 |
Phosphors
In general terms, a phosphor can be defined as a material that absorbs light at one wavelength and emits light at a different one. For example, white HB LEDs have a blue gallium nitride (GaN) semiconductor die, exciting a yellow phosphor coating made from a cerium-doped yttrium aluminum garnet (Ce:YAG) powder dispersed in a gel or adhesive-like silicone. The result is yellow light mixing with unabsorbed blue light to produce white light.
Phosphors are produced primarily in powder form and are dispersed into a silicone system, typically the same material used to encapsulate the die, at a 30% level by weight. Typical phosphor particle size is 2 to 20 µm in diameter, with a specific gravity of 4.5. These conventional phosphors have a refractive-index range from 1.7 to 2.3 for visible light. A dispersant—such as barium titanate, titanium oxide, or aluminum oxide—can be used with the phosphor.
Some factors to consider: given that all phosphors have a higher refractive index than any silicones available, one should choose the highest refractive-index silicone system available, 1.57. A two-part platinum cure system will most likely be chosen. Cure inhibitors such as sulphur, amines, and tins should be recognized in the HB LED package. Often, fluxes and die-attach adhesives contain such materials. If these materials cannot be altered, custom silicone dispersion formulations may need to be developed to compensate for inhibition.
The bond angles of the silicon-oxygen bonds create large amounts of free volume in silicone elastomers. This free volume, along with silicone’s high compressibility, bodes well for dispersions made with silicone. The more free space, the more filler that can be added, and the more uniform the dispersion. Many powder fillers are currently added to silicone systems to achieve different properties (see Table 2).
| Filler | Property | Particle size (µm) | Density | Surface area (m2/g) |
|---|---|---|---|---|
| Fumed silica | Increase strength | 0.011-0.014 | 2-5 lb/ft3 | 200-255 |
| Microballoons | Reduce density | 35-135 | 0.16 g/cc | |
| Ferro black TiO | Color | 1 0.3 | 5 g/cc 5 g/cc | |
| Boron nitride | Thermal conductivity | 7-10 | 0.4 g/cc | 13 |
| Iron oxide red | Thermal stability | 3 | 4.1 lb/ft3 | |
| Diatomaceous earth | Increase hardness | 7 | 352 g/l | |
| Carbon Silver | Electrical conductivity | 30 nm 30-40 | 6 lb/ft3 2.7 g/cm3 | 254 10 |
| YAG | White light | 2-20 | 4.5 lb/ft3 |
Consistency is the key in making a dispersion of any powder. The objective is to uniformly mix and disperse the powder particles throughout the entire liquid. The ideal dispersion breaks all of the phosphor particles down into primary particles of uniform size and separates them from each other uniformly with each particle covered by a uniform layer of silicone. Nonuniform mixing causes clumping or settling and does not provide the desired effects required from the dispersion—in this case, optimum light output. Consistency is dependent on the shearing capacity of the dispersion equipment, the length of shearing time, the liquid’s viscosity, and the powder’s particle size and density.
Typically, the maximum shear time is determined when the viscosity has plateaued or slightly decreased. The higher the particle’s surface area, the more difficult it is for the powder to disperse. More energy is needed to wet-out surface areas. Another key property affecting dispersion is the powder particle’s structure. The more highly structured the particle, the harder it is to get into the space around the particles and the more difficult it is to disperse.
ACKNOWLEDGMENTS
The authors would like to thank their staff at Lightspan Application Lab in Wareham, MA, for all the optical testing performed. MorehouseCowles, the mixing equipment division of NuSil Technology, provided its expertise in the equipment and dispersion sections of the paper.
FURTHER READING
- G. Harbers, S. Paolini, and M. Keuper, Performance of High-Power LED Illuminations in Projection Displays, Lumileds Lighting, San Jose, CA.
- D. Slater, B. Williams, and P. Andrews, U.S. Pat. 6,740,906 (May 25, 2004).
- I. Eliashevich, H. Venugopalan, B. Karlicek, and S. Weaver, U.S. Pat. 6,746,889 (June 8, 2004).
- J. Carey, W. Collins, J. Posselt, U.S. Pat. 6,590,235 (July 8, 2003).
- W. Noll, Chemistry and Technology of Silicones, Academic Press, New York (1968).
- R. Jones, W. Ando, and J. Chojnowski, Silicon-Containing Polymers, Kluwer Academic Publishers, Dordrecht (2000).
- S. Clarkson, J. Semlyen, Siloxane Polymers, PTR Prentice Hall, New Jersey (1993)
- B. Riegler, R. Thomaier, Optical Silicones for use in Harsh Operating Environments, A poster presentation at Optics East, Philadelphia, PA (Oct. 25-28, 2004).
- J. Mark, B. Erman, F. Eirich, Science and Technology of Rubber, Academic Press, San Diego (1994).
Bill Riegler is product director-engineering materials, Rob Thomaier is research director and Steve Bruner is marketing director at NuSil Technology, 1050 Cindy Lane, 93013 Carpinteria, CA; e-mail: [email protected]; www.nusil.com.