Modern techniques can fabricate IR refractive optics
Modern techniques can fabricate IR refractive optics
Proper fabrication techniques and coatings permit adaptation of difficult-to-work-with infrared optical materials to refractive optics for commercial and space applications.
Charles R. Anderson
When manufacturing optics for the infrared (IR) region of the spectrum, specifications on surface finish and figure can be considerably relaxed over those required for the ultraviolet (UV) and visible portions of the spectrum. Conversely, conventional optics made from glass, quartz, or other forms of silica are seldom acceptable in the infrared region because their long-wavelength transmission does not reach the mid- and far-infrared regions necessary to identify and quantify most molecular species. This combination forces the infrared-optics designer to use materials that satisfy some unique requirements.
Sampling optics in contact with food products for example, may be required to be on the Food and Drug Administration Generally Recognized as Safe (FDA GRAS) list. Also, very durable materials may be required for on-line sampling of extremely abrasive process streams involving, for example, additive-loaded molten polymers. Finally, spectral requirements, particularly broad-range or long-wavelength limits, may require the use of water-soluble optics.
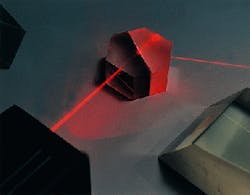
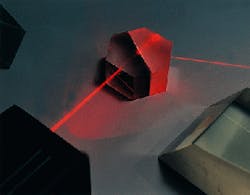
Large quantities of extremely precise infrared refractive optics made from potassium bromide, potassium chloride and cesium iodide--all much more hygroscopic and fragile than sodium chloride--are currently used in medical and laser systems and in almost all commercial infrared spectrometers (see Fig. 1). Late last year, optics made from two of the more-difficult infrared materials were part of critical space exploration programs. Cesium iodide (CsI) beamsplitter components of 1/10 l flatness are about to begin orbiting Mars aboard the Global Surveyor. In early November 1997, 1/20 l potassium bromide (KBr) optics began a seven-year journey to Saturn as part of the Cassini Mission. Both sets of optics are critical components in the mission to map the atmospheric composition of these two planets.
The manufacture of precise components from all of these materials can present challenges to an optics shop. With the correct processes and experience, however, optics with specifications similar to those placed on UV and visible silica-based optics can be fabricated.
Hygroscopic salts
Most commercial infrared spectrometers now contain only one dispersive element, a beamsplitter that is usually made from a hygroscopic material. Simultaneous requirements of broad spectral coverage, 1/10 l flatness, and 50% beamsplitter coatings over the same broad spectral region necessitate this choice. Also, many carbon dioxide (CO2) laser applications use potassium chloride (KCl), and these applications also frequently require surface figures better than 1/10 l. The traditional market for hygroscopic salts is chemical sampling systems in which the broad spectral coverage, combined with an excellent refractive index match to many nonaqueous solvents, permits construction of liquid sampling cells without the spectral interference problems caused by large-amplitude interference fringes.
Fabrication techniques for IR optics
Twenty to thirty years ago most salt optics were used as windows in chemical sampling cells, and cutting and shaping was accomplished with a wet string saw. These applications have now been superseded in importance by those requiring more-precise optics. Rough shaping of salts is now done with cutting and blocking techniques similar to those for glass, except that the slurries and lubricating and cooling agents are usually mineral oil or ethylene glycol.
Polishing is done with alumina powder and ethylene glycol on pitch or pad depending on the clear aperture and surface finish required. When an application calls for a very precise figure, the substrates should be checked prior to working under a polariscope to make sure the material is single crystal, because parts with crystal boundaries are not likely to be stable during the process and may even cleave. The final figure can also depend on crystal orientation, and this should be carefully specified to ensure consistent results. A 6:1 aspect ratio is preferred to produce the best flatness. With additional effort, however, considerably thinner parts can be produced. If figure is not critical, a water vapor polish or a mixture of ethylene glycol and water can be used to more quickly obtain a good surface finish.
Accurate determination of the figure of flat parallel surfaces in interferometric measurement systems is complicated by the fringe interference between the front and back surfaces. When working with silica-based optics this problem is easily circumvented with the use of index-matching pastes or liquids applied to the back side of the part. However, index-matching fluids that do not damage the soluble surface are not currently available. Therefore, salt components must be wedged in order to accurately measure surface figure, or, alternatively, the test technician must revert to a visual estimation of the fringe pattern.
Experienced opticians may have difficulty adapting to hygroscopic materials. Temperature and humidity must be carefully controlled in order to keep the process consistent. These materials are soft, and mistakes in processing quickly lead to loss of material. Sodium chloride (NaCl) is the easiest of these materials to work because of its hardness, while CsI is the most difficult. With patience, all can be fabricated into high-quality and somewhat-fragile optical components.
Silver halides
The silver halides are commonly used in chemical sampling systems in which resistance to water, acids, and bases is required. These materials are very soft, and optical components can be produced using hot-rolling or hot-pressing techniques. These processes are limited to thicknesses up to a few millimeters. When larger optics are required, conventional cutting and blocking techniques can be used. Good polish can be achieved using diamond powder on a pad. However, the softness and pliability limit the achievable flatness to a few waves.
It is easy to forget that many metals, particularly iron, diffuse very quickly into the silver halides and can severely compromise the spectral performance. As a result, these materials should always be worked with ceramic tooling. An additional inconvenience is the necessity of working the silver halides in a darkroom to prevent discoloration during the manufacturing process.
Semiconductors
Semiconductor materials are frequently used in infrared systems because of their unique spectral coverage and relative resistance to acids, bases, and abrasion. They can be the only choice when used for evanescent wave sampling of very opaque samples using the attenuated total-reflection technique. In this sampling technique, the very high index of refraction limits the depth of penetration and absorption of the sampling infrared beam. In imaging applications good performance can be achieved with a germanium (Ge) element combined with AMTIR as an achromat. Both germanium and silicon (Si) are traditional lens and window materials in low-power laser and imaging applications.
Good germanium optical material has become increasingly difficult to purchase because many traditional manufacturers of quality semiconductor materials have ceased production. It is imperative, therefore, to thoroughly test incoming material for free-carrier and specific-impurity absorption with either four-point probe equipment or infrared spectroscopy. Silicon of very reliable quality continues to be readily available.
These materials are cut and blocked using techniques similar to glass. Silicon is polished with diamond and oil, and the softer germanium is polished with alumina (Al2O3) and water.
Fluorides
Fluorides can be polished to a figure and finish sufficient for many UV applications. This property and their relatively low refractive index make them attractive for near-IR to mid-IR applications. Calcium fluoride (CaF2) and magnesium fluoride (MgF2) are blocked using conventional techniques and then polished with diamond and oil. The softer and slightly hygroscopic BaF2 is polished with Al2O3 and water.
If large optical components are to be produced from the fluorides, extreme care must be taken to avoid thermal shock. In particular, BaF2 is quite sensitive to thermal gradients. Testing of these fluoride parts can be done using the same techniques as for UV and visible optics.
Other IR materials
Zinc selenide (ZnSe) has now become the material of choice for lasers, imaging, and chemical sampling because of its spectral coverage and water resistance (see Fig. 2). It is, however, soft compared to the oxides, fluorides, and semiconductors. In addition, ZnSe reacts strongly with acids and somewhat less so with bases. Because of its selenium content, ZnSe is not suitable for applications involving food processing.
Thick samples of ZnSe will typically show fairly strong absorption features due to a ZnH impurity. This problem can be significantly reduced if the material is annealed after production, but it is necessary to run a high-quality infrared spectrum on ZnSe raw material prior to fabricating into parts thicker than about 10 mm. After cutting, blocking, and grinding using conventional techniques, ZnSe is polished with Al2O3 and water.
The harder materials--zinc sulfide (ZnS) and Cleartran--are on the FDA GRAS list and are less sensitive to acids and bases. They do not provide the spectral coverage of ZnSe. The Cleartran grade is normally free of impurities. Again, they can be roughed into shape using conventional techniques and are usually polished with Al2O3 and water.
The compound AMTIR can be used in combination with germanium as an achromat lens and, along with cadmium telluride (CdTe), is much more resistant to acids than ZnSe. Both of these materials are soft and brittle and are difficult to polish flat and with a good finish. A good final surface finish can sometimes be achieved by using Al2O3 and water on a silk pad.
Thallium-containing KRS-5 must always be handled with finger protection to avoid ingestion of thallium through the skin. Technicians working with this material should have frequent blood tests. The KRS-5 is a soft material usually polished with Al2O3, soap, and water. It may be possible to achieve an acceptable surface figure when polishing KRS-5 only to find that it has changed a day or two later due to its tendency to "cold flow." Guaranteeing any tight tolerance on KRS-5 can be risky at best.
Coatings
An increasing percentage of infrared refractive optics is now being produced for commercial rather than military applications. Most military applications are limited to the 3-5-µm and 8-12-µm atmospheric windows. Therefore coating design and fabrication technology have been focused on one or both of these regions. Commercial interest falls throughout the infrared from 1 to 1000 µm. Laser applications continue to grow, but the most rapidly growing markets involve broadband spectroscopy.
One of these markets, that of conventional infrared spectrometers, has experienced pressure to provide the broadest possible spectral coverage, with excellent performance extending to the longwavelength transmission limits of the substrates used for the refractive optical components. The traditional market for these spectrometers involves qualitative infrared measurements usually made on the fundamental vibrational transitions of molecules between 2.5 and 50 µm. In this region the discrimination between different compounds is most definitive. The more rapidly growing quantitative infrared market uses the overtone and combination bands found in the near-infrared region between 1 and 2.5 µm.
Instrument manufacturers have now developed instruments that will satisfy both market needs simultaneously and, therefore, require antireflection and beamsplitter coatings over both spectral regions.
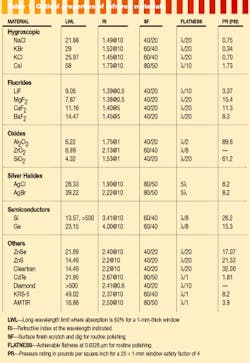
As is readily apparent in Table 1 (on p. 122), the refractive index of many of the materials used in the infrared can generate large reflection losses. When one or more refractive elements are required in a system design the overall system performance can be improved by a factor of 2 to greater than 10 with the use of broadband antireflection (AR) coatings.
These new requirements have caused beamsplitter and antireflection designs to increase in complexity from as little as 1 to 2 layers to 15 to 20 layers. Coatings have also been developed to protect the hygroscopic optical components from humidity. These coatings usually involve multiple fluoride and oxide layers, depending on how much the substrate transmission can be compromised. Coatings are also now required to protect the nonhygroscopic optics, particularly those used for attenuated total reflectance measurements. The design is required to provide good abrasion protection while being limited in thickness to a small fraction of the evanescent wave penetration depth.
The infrared-coating designer is fortunate in having several high-index materials that can be useful for producing these broad-band beamsplitters and AR coatings. The limit in coating designs is the lack of low-index materials with adequate long-wavelength transmission. Table 2 (this page) is a partial list of the materials available to the infrared-coating designer.
Most of the materials used in the infrared have properties that make them more difficult to work and/or more fragile to handle. However, when manufactured with proper techniques, coated, and handled properly, refractive infrared optics can be successfully applied to difficult earthbound and extraterrestrial applications. o
FIGURE 1. Beamsplitter made of 1/20 l potassium bromide (KBr) is used in process- control applications.
FIGURE 2. Range of infrared optical materials and shapes includes ZnSe double-roofto¥prism (1), ZnSe attenuated total-reflection plates (2-6, 11, 13), KRS-5 attenuated total-reflection plates (7 and 12), KBr 1/20 l beamsplitter (8; see also Fig.1), cubic zirconia attenuated total-reflection rod (9), ZnSe hemicylinder (10), Ge hemicylinders (14 and 15), quart¥right-angle prism (16), AMTIR roofto¥probe (17), and a ZnSe roofto¥probe (18). (Optical properties are listed in Table 1.)