VISIBLE GAS LASERS: Air-cooled ion lasers see strong biomedical demand
DONNA Z. BERNS
For many years, the air-cooled ion laser was the only available economical source of visible laser light with output power reaching tens of milliwatts. As a result, it saw significant use in OEM applications as diverse as reprographics, flow cytometry, and DNA sequencing. Recently, though, there has been competition from several solid-state lasers delivering visible light at this power level and beyond. This development spurred many to predict the imminent demise of air-cooled ion lasers or at least a major drop in demand. While many reprographics applications have switched to semiconductor lasers and diode-pumped Nd:YAG and Nd:YVO4 lasers, several high-growth applications, particularly in biomedical instrumentation, still rely on air-cooled ion lasers, and overall demand for these lasers remains strong.
Key characteristics of ion lasers
Air-cooled argon-ion lasers were first developed in the late 1970s as a simpler, low-cost alternative to water-cooled ion lasers. At that time, single-line 488-nm air-cooled lasers became an enabling technology and the source of choice in reprographic imagesetters. Compared to other laser types, they offer several advantages; some of these are inherent to the technology, and several are the result of more than 20 years of product maturation and refinement.
The air-cooled ion laser easily delivers a circular TEM00 Gaussian output beam. This beam profile can be focused to a diffraction-limited spot or beam waist, providing spatial resolution down to the wavelength of the laser light. This property, coupled with excellent beam-pointing stability and low-intensity noise, is essential in applications such as confocal microscopy and flow cytometry.
A pure TEM00 output also eliminates high-frequency noise due to beating between higher-order transverse modes, which is beneficial in fast wafer scanning and reprographic-type applications. Additionally, the beam can be easily expanded and collimated to deliver large-area illumination with a coherent wavefront, which is useful for interferometric applications.
Air-cooled ion lasers typically deliver highly linearly polarized output (with ratios exceeding 1000:1) by including a Brewster window in the laser cavity. Without this optic, they produce light with random polarization, offering the advantage of higher power at lower cost.
More-reliable tubes reduce service and warranty costs, as well as end-user downtime. Spectra-Physics (Mountain View, CA) has recently extended this advantage through plug-and-play technology slasers featuring plasma tubes with precise optomechanical alignment and registration. An end-user can replace any tube in the field without requiring a service visit or extensive optical realignment.
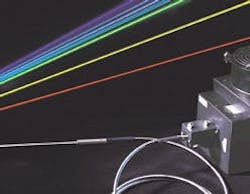
Despite these advantages, the popularity of air-cooled ion lasers is still primarily due to their output wavelength. Argon-ion devices can produce hundreds of milliwatts of output power at 457, 488, and 514 nm. Air-cooled krypton-ion lasers produce yellow and red outputs. Mixed-gas (argon and krypton) lasers can deliver all these colors simultaneously (see Fig. 1).
The 488-nm line is by far the most common, as many processes and reagents are optimized for this particular blue wavelength. Most diode-pumped solid-state lasers, in comparison, only offer a single visible wavelength—typically at 532 nm—which does not meet the requirements for most biomedical applications.
Biomedical demands true blue
Flow cytometry. Flow cytometry has long been the primary method used by both clinical and research laboratories to count blood cells and assess cell and bacterial populations. In a typical blood-cell analysis, 50 µl of blood is first treated with several reagents, each of which comprises a fluorescent molecule covalently bonded to an antibody. Each type of antibody has a high binding affinity for a specific protein antigen uniquely found on the outer surface or membrane of a particular cell type. The different reagents each emit fluorescence in a characteristic spectral band.
Once blood is loaded into the cytometer, it is forced into a narrow flow stream so that blood cells (typically 7-10 µm in diameter) pass in single file. The laser beam (or beams) focuses to an elliptical spot in this stream. Resultant Stokes-shifted fluorescence is separated by dichroic filters and detected by photomultiplier tubes. This allows counting and identifying cells according to their fluorescence intensities in the emission wavelength bands.
According to Bob Hoffman, director of instrument technology at BD Biosciences (San Jose, CA), a manufacturer of flow cytometers, his firm ships virtually all clinical instruments with a 15-mW, 488-nm air-cooled argon-ion laser. The reagents all produce Stokes-shifted fluorescence, for example, at longer wavelengths than the laser excitation. Even with the new long-wavelength fluorochromes, however, the practical limit on such fluorescence detection is just beyond 800 nm.
Hoffman explained, "We thus have a finite usable spectral bandwidth for fluorescence detection, defined by the laser at the short-wavelength end and the photodetection system at the long-wavelength limit. Short-wavelength excitation is therefore preferable because it provides the maximum spectral bandwidth for detection.
"Because each fluorophore emits over a relatively broad band, this allows the spectral resolution of the maximum number of different fluorophores in one measurement. Furthermore, the ion laser represents mature, reliable technology, which is very important given that our end users are biomedical-laboratory technicians and research biologists."
What about other light sources? Says Hoffman, "A filtered arc lamp would not have sufficient intensity. Doubled Nd:YAG (532-nm) laser excitation would excite some fluorochromes less efficiently and limit spectral bandwidth. Conversely, the helium-cadmium laser at 441 nm would provide wider detection bandwidth, but would cause problems with autofluorescence of the blood cells and would not excite the commonly used fluorophores efficiently."
Confocal microscopy. Since its introduction in the early 1980s, the confocal microscope has revolutionized biological imaging. The microscope focuses a laser beam to a small diffraction-limited spot in the sample. Resultant fluorescence (from fluorophore labels or even autofluorescence) is then reimaged onto a photomultiplier through a confocal pinhole aperture. This aperture acts as a spatial filter, passing only fluorescence that originates from the beam focus.
Fluorescence excited at other points along the laser beam is efficiently blocked, thereby providing x, y, and z discrimination. The microscope builds a complete x, y, z image by raster scanning the sample (relative to the laser spot) in the xy plane and then repeating this process at different z depths. "The workhorse laser source in our PCM 2000 microscope is the 488-nm air-cooled argon-ion laser, although many users like us to supplement this with green and red helium-neon lasers to allow excitation of multiple fluorophores," said Gerald S. Benham, manager of BioScience Confocal Systems at Nikon Inc. (Melville, NY).
As with cytometry, the 488-nm laser line is suited to exciting a number of key fluorescent labels, including DiO, acridine orange, FITC, and fluo-3, which is used for calcium imaging. The 488-nm output also works well for exciting green fluorescent protein and its cloned variants.
According to Benham, an additional practical benefit of using air-cooled argon-ion lasers is their reliability. "I can only remember two instances of having an argon laser fail in the field. Also, the low cost of this laser technology appeals to end users on tight research budgets."
Gene arrays and artificial noses
Another biomedical application benefiting from air-cooled ion lasers involves gene arrays. A gene array is a glass slide covered in a two-dimensional array of microscopic wells. Each well contains many strands of single-stranded DNA, either a single gene or small group of genes.
A large array might have some 62,000 wells, each with a different gene. Solutions containing fluorescently labeled DNA from an experiment are deposited on this array. The array is then illuminated, usually with a 488-nm argon-ion laser, and the fluorescence imaged with a wide-field microscope and charge-coupled-device (CCD) camera. The observed pattern of fluorescent dots then helps researchers characterize the unknown DNA mixture.
Illumina Inc. (San Diego, CA) recently took this concept a step further with its prototype Assembled Array technology. This technology may have potential applications as diverse as characterization of the chemistry of single cells, pharmaceutical development, and use as an "artificial nose" to detect trace chemicals. This latter is of great interest to the military, which looks for low-risk techniques to locate land mines and other unexploded ordnance.
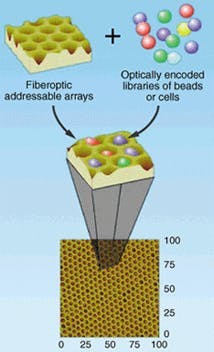
"Using a modified fiber bundle," said Illumina president John Stuelpnagel, "we can pack thousands of individual sensors into an array with a typical area of only 1 mm2." To produce an array, the firm first draws a fiber bundle to the required shape. The end is then exposed to an etching chemical that erodes the core of each fiber faster than the cladding (see Fig. 2). This creates a depression at each individual fiber. The end is then dipped in a solution containing several types of glass beads, each with a unique chemical or reagent bonded to its surface.
These beads (typically 3 µm in diameter) firmly seat in the wells. The other end of the bundle couples to a large-area CCD. The unique pattern of sensors attached to a given array is then characterized using fluorescence excited by a 488-nm air-cooled ion laser.
"Using an array containing many nonspecific chemical sensors," noted Stuelpnagel, "a system software can be taught what pattern of fluorescent dots will be produced by specific chemicals of interest, such as TNT, in our laboratory. The sensor can then be used in the field to identify these same chemicals in trace amounts."
Applications such as these demonstrate that the market for air-cooled ion lasers is thriving, despite competition. This trend is driven by the utility of the 488-nm blue output wavelength and multiple wavelength flexibility, coupled with reliability and cost-effectiveness.
Donna Z. Berns is a marketing manager at Spectra-Physics, Mountain View, CA 94039; e-mail: [email protected].