Medicalwatch: OCT moves closer to broad-based commercialization
Optical coherence tomography (OCT) continues to be one of the few light-based technologies gaining a commercial foothold in the medical field. And if Coherent Diagnostic Technology (CDT; Westford, MA) is successful in its efforts to commercialize technology licensed from the Massachusetts Institute of Technology (MIT; Cambridge, MA), OCT could well become a standard diagnostic tool for multiple medical applications.
Optical coherence tomography offers several advantages over existing diagnostic technologies. It is optically based, noncontact, and achieves resolution in the 2-10-µm range—one to two orders of magnitude greater than ultrasound or other tomographic imaging technologies such as computed tomography or magnetic resonance. In addition, it precludes the need for surgical biopsies.
Simply put, OCT involves directing infrared light waves onto an object, organ, or tissue specimen and measuring how long it takes for the light to return. The basic OCT system works by gating out the reflections from different depths in the tissue or material using an interferometer. The reflected signals from the sample interfere with the reference signal only when they emanate from a depth in the sample that is identical to the length of the reference arm. This reference depth is determined by the position of the retroreflecting mirror.
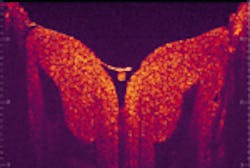
By moving the mirror once the interferometer has extracted the optical signals from the reflected light, the data are processed and high-resolution, cross-sectional (that is, 3-D) digital images of microstructures are produced on an attached computer monitor—all in a matter of seconds. And, because the resolution is so high, the process reveals an enormous amount of data, with the possibility of imaging at the cellular level (see figure). "Optical coherence tomography allows physicians to immediately go beneath the surface of what they are looking at," says Paul Magnin, president of CDT.
Coherent Diagnostic Technology was founded in Feb. 1998 by three of the leading developers of OCT: Eric Swanson, group leader of the optical communications technology group at MIT Lincoln Laboratory; James Fujimoto, professor of electrical engineering and computer science at MIT; and Dr. Mark Brezinski, assistant in medicine at Massachusetts General Hospital, professor at Harvard Medical School, and visiting professor in electrical engineering at MIT. Day-to-day operations are handled by Magnin, who spent 17 years at Hewlett-Packard prior to joining CDT, most recently as general manager of the imaging-systems division.
MIT holds the fundamental OCT patents but has licensed exclusive rights to this technology to CDT—with the exception of ophthalmology. Those rights have been licensed to Carl Zeiss (Jena, Germany), whose Humphrey Instruments subsidiary (San Leandro, CA) has been selling an OCT imaging device for diagnosing macular degeneration since 1996. Another OCT company founded by Swanson and Fujimoto, Advanced Ophthalmic Devices, was acquired by Humphrey in 1994. In fact, Zeiss has held a minority equity interest (slightly less than 50%) in CDT since its inception and is considered CDT's microscopy partner, with rights to microscopy applications of the technology. At present, CDT is valued at $30 million, and additional partnerships worth between $2 million and $6 million each are in the works.
Additional financial support has come from another significant source in the past year. Coherent Diagnostic Technology won a $1.8 million Advanced Technology Program award from the US National Institute of Standards and Technology last October that has accelerated much of the company's technology development over the last 12 months. The company plans to continue refining its OCT technology to improve performance and increase the number and type of applications. Technical improvements are expected to include new high-power, high-resolution optical sources; low-cost video-rate scanning modules; advanced signal-processing algorithms and hardware; and new probe modules, including catheters, endoscopes, and hand-held units.
Two-tiered approach
With the MIT technology and engineering expertise under its belt and some significant development capital in its pockets, CDT is taking a two-tiered approach to commercializing its OCT system. First, CDT is developing a broad technology platform that can be easily customized for a variety of applications, allowing the company to take advantage of economies of scale. In addition, the company also plans to partner with large, established medical-device companies in specific markets to gain better access to those markets.
Initially, CDT will target three medical markets: cardiology, gastroenterology, and microscopy. Additional medical applications on the horizon include dermatology and dentistry; potential nonmedical uses include nondestructive testing and on-line process control for manufacturing.
Coherent Diagnostic Technology's first commercial product will likely be a nonsurgical microscopy OCT device, primarily because it does not require US Food and Drug Administration (FDA) clearance. Initially designed for use in genetic analysis and similar applications, this product is also being developed for microscopic surgery.
Nonmedical applications
An engineering prototype has been used in clinical testing for microscopy. Although earlier versions were laser-based, the current device uses a wide-bandwidth, low-coherence infrared source (1320 nm) that is not a laser, according to Magnin. Energy is delivered to the sample via a thin fiberoptic cable with a microlens at the tip; because the system is fiberoptics-based, it can be easily interfaced to standard medical-imaging devices such as catheters, endoscopes, and laparoscopes. The prototype has a high-speed proprietary reference scanning technology that is capable of imaging at 30 frames/s. It is interfaced with a Zeiss Stemi SV-11 microscope so that when the visible image is in focus through the scope, it is also in focus on the system monitor.
"The [nonsurgical] microscopy application has the advantage of something we can do very quickly without the need for regulatory approval," Magnin says. "We expect to have the nonmedical microscopy product out and available in less than nine months."
Next on the agenda is surgical microscopy; preclinical trials are expected to begin early next year. But CDT believes there is a significant market for a product that can provide a better and earlier diagnosis of heart disease and various throat and stomach cancers. According to Magnin, human clinical trials of these two applications will likely commence by the end of next year.
In the meantime, CDT is working to establish several key industry partnerships. While Magnin would not disclose who these potential partners are, he did say that the company's cardiology partner would probably be in place by the end of year.
About the Author
Kathy Kincade
Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.