Video microscopy gains nanoscale access
Cooperation between academia and industry results in new visualization techniques for microscopy and cell biology.
Kenneth Kaufmann
Electro-optic imaging has an indispensable tool for studying cell biology. Using optical imaging devices ranging from a chalnicon (high-performance vidicon) tube or cooled charge-coupled-device (CCD) microscope camera to a photomultiplier tube in a confocal scanning microscope, re searchers can observe structural and molecular phenomena in living cells that a few years ago would have been impossible. The broad dynamic range of modern detectors, coupled with image-processing ad vances, are allowing microscopes to have increased contrast, resolution, magnification, and visibility. Images can now be detected faster and with greater sensitivity.
Twenty years ago, most of the technology to permit such performance did not exist. By today`s standards, data acquisition and computer systems were very crude and expensive. Vidicon cameras used in microscopy were relegated to education, allowing a group of students to simultaneously study the same slide by viewing its image on a monitor. Film was the preferred method for image capture in microscopy because it achieved higher resolution.
By the late 1970s, video technology had advanced to where it could be considered for use in microscopy. Since then, the development of three visualization techniques, along with confocal laser scanning microscopes, have virtually brought about an electronic revolution in microscopy. The techniques are Allen video-enhanced contrast differential-interference contrast (AVEC-DIC), Allen video-enhanced contrast for polar ization microscopy (AVEC-POL), and digital subtraction. Their development illustrates how partnership be tween research scientists and engineers can advance technology.
Video imaging
Many researchers, especially Shinya Inoue of the Marine Biological Laboratory (Woods Hole, MA), contributed to the early development of video microscopy, but perhaps the most striking example is the collaborative work of Robert and Nina Allen at Dartmouth University (Hanover, NH) and engineers at Hamamatsu Photonics (Bridgewater, NJ). Together, in the late 1970s, they created an entire field of activity in a very short time. The Allens suggested that advances in video cameras might make them useful not just for teaching, but for also for biological research. As a trial, in 1977 they borrowed a Hamamatsu C1000 computer-compatible video camera that had 1000 ¥ 1000-pixel resolution. Initial results showed that the camera had promise but, because there was no funding to buy it, the Allens returned it.
Then in 1978, the C1000 camera was borrowed again, for use in a microscopy course at the Marine Biological Laboratory at Woods Hole. The Allens understood that while the human eye could not discriminate the details of the cell structure in the presence of high background light levels, the vidicon tube in the camera could. A differential-interference contrast mode of imaging was chosen as a test case. The Allens requested that the beam current be increased so that the camera could operate at higher background levels. This camera was equipped with a chalnicon tube, which had a beam current greater than the 200-nA current standard at the time for other types of vidLFW9611. The Allens also requested that the video offset be raised. For the C1000, this was easily accomplished by turning a potentiometer, a feature not on other contemporary cameras.
The video offset feature subtracted a constant dc level from the video signal, effectively subtracting part of the background. The signal from the specimen relative to the signal from the background could thus be amplified without saturating the monitor. At the first attempt, this technique did not seem to work, but while the Allens watched the video monitor, Hamamatsu engineer Hitoshi Iida adjusted the beam current, probably exceeding the recommended level. Suddenly, the cell structure appeared with a degree of contrast never before seen by the human eye. The vidicon had stretched the optical signal, which greatly enhanced the contrast.
Back in the Allens` laboratory, Jeff Travis and other investigators quickly applied this increased video contrast to polarization-contrast microscopy. It was then observed that contrast improved when bias was retarded to levels that caused conventional polarization contrast to fail because the background became too bright. With the help of Huseyin Yilmaz, a consultant to Hamamatsu Photonics, the Allen team was able to develop the theoretical equations that explained the AVEC-POL technique.
Theory of this video imaging method was published in 1981, along with a description of the AVEC-DIC method.1 Parallel work performed by S. Inoue at the Marine Biological Laboratory produced similar discoveries at almost the same time--illustrating how once technology reaches a certain level of development, it can result in simultaneous discoveries.
The images obtained 18 years ago with that camera had greatly enhanced contrast, but they did not reproduce very well because the only way they could be copied economically was by photographing them from a video monitor. Back then, video tape was a much clearer way of viewing the images than the grainy photo graphs taken of the video monitor, so the video signals were recorded on video tape for further analysis and teaching. To day, with modern digital image-processing systems, it is possible to obtain much better prints of the images from those same videotapes (see Fig. 1). The early camera, no longer in use, is in the laboratory of Robert Allen`s widow, Nina Allen, professor in the botany department and director of the Cellular and Molecular Imaging Facility at North Carolina State University (Raleigh, NC).
Modern enhancements
The improved contrast obtained with the AVEC method revealed previously undetected lens defects that caused dark blotches (mottle) in the image. In addition, the vidicon tube did not have uniform spatial sensitivity (shading). These effects limited image quality. One day, as Nina Allen was trying to manually subtract a blank image from a cell image using photogra phic negatives, Hitoshi Iida stopped by and asked what she was doing. As she explained it to him, he realized that a newly developed video frame memory developed by Hamamatsu for streak-camera digital readouts could work in this application as well.
Just as the Allens had borrowed the C1000 camera from Hamamatsu, they borrowed the video frame memory for their experiments and saw immediately that digital subtraction of the remaining background greatly improved the image. The enhancement even allowed them to see objects thought to be smaller than the maximum resolution of the instrument (see Fig. 2). They could now observe single microtubules (25 nm), intermediate filaments (10 nm), synaptic vesicles (30 to 50 nm), and coated vesicles (30 to 100 nm) in motion within living cells, such as squid axons.2
These revelations yielded a new field of cell biology and have been responsible for major discoveries such as the work in 1983 on the protein kinesin, the "molecular motor" protein that moves some vesicles on microtubules and is involved in mitosis, the exact division of chromosomes to daughter cells. Only through the cooperation of academic scientists and industrial engineers were these advances possible. At companies such as Hamamatsu Photonics an active program of collaboration with academics throughout the world is continuing in search of technology breakthroughs. o
ACKNOWLEDGMENTS
The author wishes to thank Nina Allen of the Department of Botany, North Carolina State University (Raleigh, NC), for providing the figures in this article, which were recently digitized from video tapes taken nearly 20 years ago. Thanks also to Hitoshi Iida of Hamamatsu Photonics for his assistance in preparing this article.
REFERENCES
1. R. D. Allen, J. L. Travis, N. S. Allen, and H. Yilmas, Cell Motility 1, 275 (1981); R. S. Allen, N. S. Allen, and J. L. Travis, Cell Motility 1, 291 (1981).
2. R. D. Allen and N. S. Allen, J. Microsc. 129, 3 (1983).
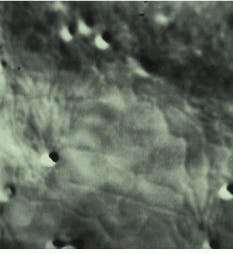
FIGURE 1. Micrograph of a small section of an onion epithelial cell demonstrated the ability to image the endoplasmic reticulum in living plant cells. It was one of the earliest specimens imaged using the frame memory diffraction method. The cell was recorded using the AVEC-DIC method; mottle (dark blotches) was removed from the raw image by background subtraction, allowing clear visualization of the dynamics of a living plant cell. The image seen here was recently digitized from the original video tape, then printed by a digital printer.
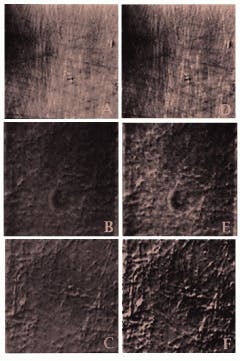
FIGURE 2. Micrographs made from 18-year-old video tapes, shown here in pairs for easy comparison, are some of the first images of isolated brain-cell nuclei microtubules. Both AVEC-POL and AVEC-DIC visualization techniques were used. Individual microtubules made visible by polarized light and video enhancement (A) are seen more clearly when the image is digitized and computer-enhanced (D). The raw image of these microtubules (B) is also sharper when computer-enhanced (E). Further clarity of the microtubules is obtained when the video frame memory is used for background subtraction of mottle imperfections (dark blotches) inherent in the microscope system (C); this digitized image has been further enhanced (F). The revelation that these and other large biological molecules could be clearly imaged in the dynamic state led to development of video assays of microtubule movements and to the discovery of the motor protein kinesin.
KENNETH KAUFMANN is in the marketing department at Hamamatsu Corp., 360 Foothill Rd., BOX 6910, Bridgewater, NJ 08807-0910.