Fluorescence microscopy of labeled biomolecules is an important technique for the study of many cellular processes. By chemically attaching a fluorescent-dye molecule to a microstructure or compound of interest and exciting it with an appropriate laser, it has become possible to watch biochemical changes as they occur inside living cells. Some observers credit the instrumentation developments with revolutionizing light microscopy (see photo).
This revolution is a direct result of the increased spatial resolution of several new, laser-based imaging techniques.1 By only looking at light generated in a small sample volume, both confocal and multiphoton laser scanning microscopes can image microstructures located inside individual cells. On the one hand, confocal microscopy restricts the imaged volume by spatially filtering the fluorescence from a laser-illuminated sample as it returns to the image plane. On the other hand, multiphoton spectroscopy achieves high spatial resolution by localizing the excitation to a very small volume at the instrument focus.
In addition to their inherently high resolution, multiphoton excitation techniques are capable of exciting short-wavelength fluorescence with infrared (IR) light. Because the excitation produced by an IR-emitting, modelocked laser is localized to the instrument focus, damage to the sample is also minimized. When compared to direct ultraviolet (UV) excitation in a confocal microscope, other advantages of multiphoton excitation include reduced optical aberrations and background fluorescence, lower scattering losses, and the comparative robustness of commercially available excitation sources.
This article describes the basic operating principles of a multiphoton excitation microscope, relevant hardware issues, some representative applications, and possible directions for the further development of new laser sources for this imaging application.
Multiphoton excitation is highly localized
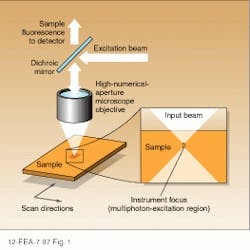
The unique advantages of multiphoton excitation microscopy with respect to single-photon techniques are directly related to the physics of the excitation process itself. In an epi-illuminated scanning fluorescence microscope, the sample of interest is excited by a laser beam that is focused to a small, diffraction-limited spot with a high numerical-aperture objective (see Fig. 1). Light within the sample is brought to a tight focus for a very small distance determined by the numerical aperture of the objective and the wavelength of the excitation. Fluorescent light emitted from the excited sample volume is collected by the same lens, separated from the excitation beam using a dichroic reflector, and imaged onto a suitable detector.In the two-photon excitation process, fluorescence results from the simultaneous absorption of two photons at a rate that is proportional to the square of the laser intensity. The constant of proportionality is such that significant emission requires intensities much higher than those required for single-photon processes. Fortunately, these intensities are easily produced by the tightly focused output of a modelocked Ti:sapphire laser operating at an average power of a few tens of milliwatts.
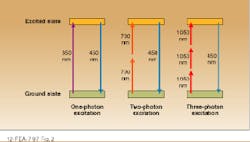
Because the intensity falls off as the square of the distance from the beam waist in such a system, two-photon emission is confined to a small region near the focus. As a result, spatial resolutions comparable to those obtained with a confocal microscope can be obtained without inserting a spatial filter into the system. Because two photons contribute to the excitation, the wavelength of the excitation source is typically much longer than that of the emission; that is, UV transitions can be excited with IR lasers. In the case of NADH, 450-nm fluorescence is produced by focusing the output of a 700-nm, modelocked Ti:sapphire laser into the sample.
Three-photon excitation has also been observed from several important UV-emitting dyes with excitation wavelengths in the 960- to 1050-nm range. Experimental studies indicate that the same emission intensity can be achieved with two- and three-photon processes, although higher powers are required in the latter case. A theoretical analysis indicates that the limiting spatial resolutions are comparable for both techniques.2
In addition to high spatial resolution and reduced sample damage, multiphoton techniques are uniquely capable of looking at processes deep within a sample. The reduction of excitation intensity by sample scattering limits single-photon confocal microscopy to the sample surface. Because scatter and sample damage are both reduced at IR excitation wavelengths, two-photon excitation can be used to image structures that lie hundreds of microns below the sample surface.
Modelocked lasers are required
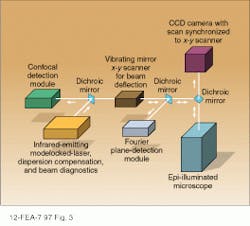
Because of the high intensities and wavelengths required, modelocked dye and Ti:sapphire lasers are ideal excitation sources for multiphoton laser scanning microscopy. In the system in Fig. 3, the output of an argon-ion-laser-pumped, modelocked Ti:sapphire laser with a pulsewidth of approximately 100 fs is imaged onto the sample using an objective lens with a high numerical aperture.3 Depending on the pulsewidth of the source and the dispersion of the objective lens, a dispersion compensator is sometimes placed between the modelocked laser and the objective. For quantitative measurements, a knowledge of the detailed pulse characteristics is provided by monitoring the dispersion-compensated laser output with a suitable autocorrelator (see Laser Focus World, Sept. 1997. p. 99).To generate a two-dimensional image of the sample, the focused spot is scanned in a plane perpendicular to the excitation beam with a set of oscillating mirrors. Moving the focused spot along the beam direction allows the depth of the scanning plane to be moved to different depths within the sample, ultimately yielding a three-dimensional picture.
Multiphoton techniques are compatible with a number of detection techniques, three of which are shown in Fig. 3. Suitable dichroic mirrors are used to separate the fluorescence emission from scattered excitation light and direct it to the appropriate detection channel. By focusing the fluorescence output onto a CCD camera and synchronizing the scan rate of the camera with that of the excitation beam, the two-dimensional sample image can be viewed directly. Descanned confocal detection is achieved by traversing the x-y scanner in a direction opposite to that of the input beam and placing a pinhole in front of the detector.
Maximum sensitivity and resolution are achieved by using a Fourier detection technique in which the back aperture of the objective is imaged directly onto a photomultiplier tube. In this case, losses associated with descanning the image are avoided, resulting in higher sensitivity.
Researchers at Cornell University (Ithaca, NY) used an objective lens with a numerical aperture of 1.3 and a 100-fs modelocked Ti:sapphire laser operating at 80 MHz and determined the theoretical powers needed to saturate two-, three-, and four-photon processes at 30, 150, and 300 mW, respectively. Using the same objective and a 700-nm source, they calculated that resolutions of
0.6 and 0.2 µm in the directions perpendicular and parallel to the sample plane, respectively, were possible for a two-photon excited sample.
Watching calcium waves
The calcium ion (Ca2+) is important in biological systems because it is released by intracellular structures as part of many metabolic processes. By ‘watching’ the release of this ion as various structures are stimulated, biologists gain important knowledge on how the cell works. Ratiometric calcium indicators such as Indo-1 change their emission wavelength when bound to Ca2+, making it possible to quantitatively determine ion concentrations from scanned fluorescent images. In practice, the fluorescence power emitted by the sample is simulataeously measured at two different wavelengths and the ratio computed.
Illumination with 345-nm light can be used for single-photon excitation but quickly damages living cells. Two-photon excitation has reduced this problem and provided dynamic images of calcium activity.
When stimulated by electrical impulses, calcium ions are released by the cellular membrane and/or internal structures. Viewed in time, the ion concentration is variable and can be spatially localized into ‘waves.’ Using Indo-1 and two-photon excitation, Watt Webb and collaborators at Cornell have investigated the kinetics of calcium release ion several different cells, recording line images of concentration transients with millisecond resolution.3
In other work, Winfried Denk (Bell Labs; Murray Hill, NJ) has used a scanning multiphoton microscope to look at processes inside the brain of living rats.1 As described above, the reduced scatter and tissue damage associated with IR two-photon excitation makes it possible to look at stuctures well below the sample surface. The brains of live rats were stained with a calcium indicator. Then the activity at the synapses between brain-cell neurons that were stimulated by tickling the rat`s whiskers was imaged in planes that were well below the brain surface.
Diode-laser-based microscopes
While conventional argon-ion and Nd:YAG laser-pumped systems have been used for most of the published experimental work on multiphoton microscopy, it is obvious that diode-based sources can be used to good advantage. In the short term, we can expect to see CW intracavity-doubled, Nd:YVO4 and Nd:YAG lasers replacing argon-ion lasers as pump sources for modelocked Ti:sapphire and dye lasers (see Laser Focus World, Nov. 1997, p. 91). In addition to eliminating the need for a dedicated cooling line, the use of diode-pumped sources will lead to improved stability and reduced size.
Ultimately, frequency-doubled, modelocked diode lasers may replace Ti:sapphire and dye lasers in many applications. While the tuning range of a semiconductor laser is narrower than more conventional technologies, there are many applications outside of the research laboratory in which broad tunability is not a requirement.
For example, most of the current commercial applications of fluorescence microscopy are based on air-cooled argon-ion lasers and a set of dyes that are well-matched to their emission lines. Modelocked diode lasers offer an improvement over this situation because their output wavelengths are not restricted to a few discrete transitions.
Comparing the average power and pulse-length requirements for two-photon microscopy with the published output parameters of state-of-the-art, modelocked diode lasers, it is apparent that suitable devices have already been demonstrated. For example, Peter Delfyett (CREOL; University of Central Florida, Orlando, FL) has demonstrated pulsewidths shorter than 200 fs at 850 nm with average powers greater than 10 mW.
Further progress in the development of modelocked diodes having output wavelengths matched to common dye molecules could eventually lead to the development of multiphoton excitation systems with commercial applications. In addition to high-resolution microscopy, such a source would find applications in flow cytometers and other fluorescence-based instruments.
Although the development of diode-based devices appears straightforward, attempts to develop a solid-state replacement for the air-cooled argon-ion laser have shown that it is often easier to see the path to commercialization than to actually travel it. Hopefully, the wait for a modelocked diode laser won`t be as long as it was for an air-cooled argon-ion laser replacement.
REFERENCES
1. T. Gura, Science 276, 1988 (1997).
2. C. Xu et al., Proc. Natl. Acad. Sci. 93, 10763 (1996).
3. R. M. Williams, D. W. Piston and W. W. Webb, FASEB Journal, 8, 804 (1994).
G. J. Dixon | Contributing Editor
G. J. Dixon was a Contributing Editor for Laser Focus World.