JÖRG FRISCHEISEN, NILS A. REINKE, and WOLFGANG BRÜTTING
Organic light-emitting diodes (OLEDs) have received much attention over the past several years, having been demonstrated as promising candidates for general lighting and display applications. A typical OLED structure has a total thickness of only 100 to 500 nm and consists of several organic layers (small molecules or polymers) sandwiched between two electrodes. By applying a voltage across the device, electrons and holes are injected into the device and then recombine in the emitting layer, generating photons.
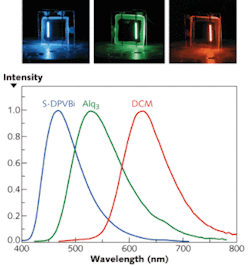
OLEDs are area emitters with a broad emission spectrum that depends on the material of the emitting layer; their peak wavelength can be made to fall anywhere within a wide spectral range (see Fig. 1). Emission of white light with a very high color-rendering index is achievable by combining different OLED materials. Besides their high efficiency and color tunability, OLEDs offer the advantage that, unlike their inorganic counterparts, they do not require epitaxial film growth on crystalline substrates. They can be produced not only on glass substrates, but also on flexible substrates such as plastic or metal foils, leading to many new innovative applications.
Application of OLEDs in sensors
Because of their ease of processing and deposition on many different substrates, OLEDs are perfectly suited to use as integrated light sources in many sensing applications.1-3 Here, we present a novel surface-plasmon-resonance (SPR) sensor based on an integrated OLED light source. Surface-plasmon-resonance sensors detect changes in the refractive index of a dielectric medium that is adjacent to a thin metal film.4 Excitation of surface plasmons is usually accomplished in the regime of attenuated total reflection when p-polarized light is incident through a glass prism and onto the metallic sensing layer.5
The metal film’s typical thickness of only a few tens of nanometers allows the evanescent field of the reflected light to excite surface plasmons at the opposite metal/dielectric interface. The SPR can be observed as a minimum of the reflected light intensity either as a function of the incidence angle or the wavelength—changes the refractive index in the medium adjacent to the metal film can be detected by shifts of the SPR position. Surface-plasmon-resonance sensors are widely used to monitor biospecific interactions or to determine concentrations of biomolecules in a solvent.
In recent years there has been growing commercial interest in SPR sensors, and especially in developing compact devices. However, the light source still remains a stumbling block in the development of small SPR sensors. Usually, SPR spectroscopy relies on bulky sources with high power consumption, such as lasers or halogen lamps. Consequently, direct assembly of an integrated light source onto a monolithic SPR sensor device is desirable for the next step in sensor miniaturization.
Integrated OLED source
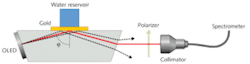
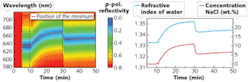
Our research group at the University of Augsburg invented an SPR sensor based on a prism and an integrated OLED light source (see Fig. 2).6 The light emitted by the OLED leaves the prism after reflection at the sensing layer and passes a linear polarizing filter before it is focused by a collimating lens onto an optical fiber guiding it to a spectrometer. The basic functionality of the sensor was demonstrated by measuring the spectral and angle-dependent surface-plasmon dispersion at metal/air interfaces for sensing layers consisting of silver and gold with different thicknesses.7For the first five minutes, the water reservoir next to the gold film was empty; thus we were measuring the reflectivity of a gold/air interface at an angle of incidence far from that for the resonance position. As a result, no minimum was observed and the reflectivity of the gold film was very high over the whole wavelength range. After adding 4.5 ml of pure water (an amount sufficient to cover the gold film completely with water), a pronounced minimum appeared. The lowest reflectivity was located at a wavelength of about 634.5 nm. After 10 minutes, 0.5 g of NaCl was added; after one more minute, stirring inside the water reservoir was started. The salt slowly dissolved and the minimum position shifted to higher wavelengths with increasing salt concentration. After 30 minutes we added 5 ml water. This dilution instantaneously reduced the concentration of NaCl and the minimum position moved back to lower wavelengths.
The refractive index of water and the concentration of dissolved sodium chloride (cNaCl, in wt.%) can be deduced from the spectral position of the minimum. The increase of refractive index with NaCl concentration is well-described by the following equation:
n = 0.00177cNaCl + 1.3329
The maximum measured NaCl concentration was 10.8 wt.% and dropped to 5.5 wt.% after the dilution (see Fig. 3, right). The theoretical concentration derived from the amount of inserted water and salt was 10 wt.% before and 5 wt.% after the dilution, respectively. The difference between the measurement and the expected value possibly resulted from the evaporation of water during stirring. This could also explain the slow increase over time after the dilution. In our setup, the sensor resolution was approximately 1 nm and the detection limit for the concentration of NaCl was about 0.3 wt.%.
This experiment clearly demonstrates that the sensor is capable of monitoring the concentration of dissolved NaCl in water in real time; thus, the sensor also has the potential to measure concentrations of other materials or biomolecules within a solvent and can be used for the analysis of protein interactions or adsorption processes. For such measurements, the detection limit could be greatly increased by using a “functionalized” gold surface (one with active organic molecules attached). In addition to measuring the dissolving process of NaCl, the sensor was successfully used to monitor temperature changes inside a water reservoir.8
Benefits of OLED-based sensors
The resolution of our prototype sensor can be further increased by reducing the signal-to-noise ratio, for instance by operating the OLED at a higher brightness. Because the OLED has a spectrally extended emission, it is not possible to achieve the same resolution and detection limit as a device with a laser light source. However, we want to emphasize that in contrast to SPR devices based on a laser, our sensor utilizes a fully integrated light source. Thus, there is no necessity to couple light from external bulky sources into the device, which makes the sensor particularly interesting for miniaturization and for low-cost applications. An additional benefit of using an OLED is that the active area can easily be adjusted to any shape required for a specific application. Furthermore, a white OLED would allow the sensor to cover a larger spectral range, making it possible to use the sensor in a wide range of applications.
We anticipate that our SPR configuration can be monolithically integrated on one common substrate, thus making it unnecessary to use a prism. By using an organic photodetector rather than a spectrometer, it should be possible to produce an all-organic, disposable SPR sensor that has a tremendous potential for miniaturization.
ACKNOWLEDGMENTS
The authors wish to acknowledge financial support by the Elite Network of Bavaria through the international graduate school “Materials Science of Complex Interfaces” as well as OSRAM OS for providing us with some of the OLED materials.
REFERENCES
- L. Bürgi et al., Org. Electron. 7, p. 114 (2006).
- X. Wang et al., Proc. SPIE 6036, p. 60361O (2005).
- M. C. Gather et al., Adv. Mater. 20, 1966 (2008).
- J. Homola and S.S. Yee, Sens. Actuators B 37, p. 145 (1996).
- H. Raether, Surface Plasmons on Smooth and Rough Surfaces and on Gratings, (Springer-Verlag, Berlin, 1988), Chap. 2.
- N.A. Reinke et al., German Patent Application, No. 10 2007 021563.2 (2007).
- J. Frischeisen et al., Proc. SPIE 7003, 70031B (2008).
- J. Frischeisen et al., Optics Express 16, p. 18426 (2008).
Jörg Frischeisen is a Ph.D. student and Wolfgang Brütting is a professor at the University of Augsburg, Institute of Physics, Universitätsstrasse 1, 86159 Augsburg, Germany; e-mail: [email protected]. Nils A. Reinke is a lecturer in physics at the Institute of Computational Physics, Zurich University of Applied Sciences, PO Box 805, 8401 Winterthur, Switzerland.