XAVIER LEVECQ and JORDI ANDILLA
The development of new microscopy techniques has become an area of intense interest, in particular due to its impact on biomedical applications, including pathology detection, pharmacology, and treatment evaluation. While technical advances in lasers, microelectronics, and optoelectronics have provided the foundation for important developments in fluorescence, nonlinear, and scanning laser microscopy, further improvements were needed to increase resolution and contrast while simultaneously limiting the damage—such as photobleaching—caused by the imaging itself.
Even with these novel techniques, the optical aberrations introduced by the system and the specimen continue to affect image quality. In the case of life-sciences microscopy, compensating for optical aberrations was, until recently, a privilege reserved for a select few with knowledge both in both optics and in their primary area of expertise.
One potential solution to overcoming the limits of any microscopy technique is adaptive optics (AO), a technology originally developed for astronomy to improve the vision of ground-based telescopes. Soon after the introduction of AO in the early 1990s, scientists in other domains, notably microscopy, saw the technology's potential to help them circumvent the limitations caused by similar circumstances.
The goal is to increase resolution and contrast, while at the same time reducing damage to the sample. In one of its most recent incarnations, AO has been used by researchers to compensate for both the inherent aberrations caused by the optical setup and the dynamic aberrations caused by the specimen itself. For convenience, this ensemble (optics plus specimen) will be called the "optical system."
Adaptive optics has been implemented into numerous microscopy techniques ranging from simple widefield fluorescence to nonlinear approaches such as two-photon excitation fluorescence (2PEF), second- and/or third-harmonic generation (SHG/THG, respectively), and coherent anti-stokes Raman spectroscopy (CARS), and on to confocal microscopy and optical coherence tomography (OCT).
Research from around the world has demonstrated how AO can improve image quality in advanced microscopy. For this reason, it is urgent that AO be expanded beyond its use in physics laboratories to wider-scale bioimaging applications. To achieve this, a standard "AO box" that can be used as an accessory for any microscope is necessary.
Such a tool must work with the wide variety of microscopy techniques available today. In addition, the fact that each microscope manufacturer has its own particularities (configurations, focal distances, and individual element sizes) must be taken into account.
To these ends, we have developed a "turnkey" device, based in a single box plugged into the CCD port of the microscope, which enables users of nearly any standard commercial microscope to implement AO into their systems.
Using AO in microscopy
Efforts to improve image resolution and contrast in microscopy are generally in vain if aberrations in the optical system degrade image quality. These aberrations can be divided into two primary categories: those introduced by the optical system's components, and those induced by the sample and the immersion media. The first are primarily static and can generally be limited by using good material and performing precision optical alignment of the microscope's hardware components. However, the latter are intrinsically dynamic. Of the many factors contributing to the latter, the nature of the sample and the depth of the object plane within the sample both weigh heavily.
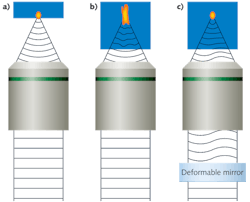
In a high-quality 3D scanning microscope, we can assume that almost no aberrations are present when imaging the first surface of the sample, where a diffraction-limited spot is produced (see Fig. 1a). However, when focusing the scanned laser spot deeper into the sample, an index mismatch between the immersion media and the sample occurs due to the heterogeneous nature of the sample's index of refraction; this in turn produces an aberrant spot that reduces the contrast and resolution of the resulting image (see Fig. 1b). Using an active optical element such as a deformable mirror (DM), we can compensate for the deformations produced by the sample and recover a diffraction-limited spot (see Fig. 1c).
The same reasoning can be used for the microscope's imaging path. If we consider the emitter as an ideal source, then the aberrations produced by the sample will enlarge the focal spot's size and contrast in the resulting image will be reduced. In fact, the integration of AO for microscopy can be performed on the imaging path, on the excitation path, or on both simultaneously. For example, AO in the imaging path might be used with standard widefield fluorescence microscopy, in the excitation path with 2PEF, SHG, or THG, or in both paths for confocal microscopy.
Plug-and-play AO
Providing for the needs of users in all three cases presents interesting challenges. First, the right components for wide-ranging compatibility must be selected and tested. Because the keystone of any AO system is the DM, one with excellent optical qualities and wide corrective ability has to be chosen. Then, to obtain best results, the DM must be optically conjugated with the optical system's pupil, which in this case is the microscope objective. However, the relative apertures of biological objectives from major microscope manufacturers are unique to each model (see Fig. 2). In order to adapt to the properties of the maximum number of objectives as possible, a motorized system must be included that conjugates and scales the pupil of the objective with the DM, all the while maintaining the scale of the resulting image (magnification of the image must remain unchanged for any corrected aberration).Imagine Optic developed what we call Micao to meet these needs (see Fig. 3). This consists of an all-in-one box accessory that can be placed in the optical system by plugging the device into the camera port between the imaging system and the microscope. Using the microscope's camera port ensures compatibility with most commercial microscopes; however, the design of Micao anticipates using an extra lens to adapt the device to custom microscopes. The DM has a large stroke and high optical quality, and complies with the IEC standards 61010 and 1236 for electrical safety and electromagnetic compatibility.
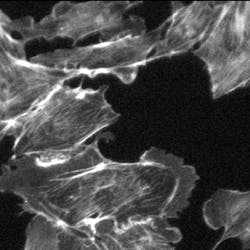
An example demonstrates the improvement in contrast of an image of bovine pulmonary-artery endothelial (BPAE) cells in the presence of spherical aberrations that are caused by the index mismatch between the immersion medium and the sample (see Fig. 4).
Adaptable AO
Adaptive optics can be implemented in two ways: the more common "closed-loop" configuration, in which a wavefront sensor is used to measure aberrations; or the increasingly popular open-loop (or "sensor-less") configuration, in which complex algorithms, along with user intervention, allow for merit-based selection of correction criteria. Closed-loop AO offers the advantage of rapid aberration compensation by introducing an artificial guide star into the specimen. That second source enables the wavefront sensor to measure aberrations and send commands to the DM very quickly, but has the often unwelcome side effect of increasing photobleaching or other forms of sample degradation. The iterative algorithms used in open-loop AO allow the user to correct for aberrations without the use of a second source. Micao's design encompasses both correction strategies.
Software integration is also essential. A large array of software is available to biologists and, although device-specific software is usually provided by the microscope manufacturer, some manufacturers choose to use Metamorph (Molecular Devices; Sunnyvale, CA), µmanager (open source, developed by Vale Lab, University of California–San Francisco), or one of the many other titles available. To ensure compatibility with the widest possible range of hardware/software configurations, the software interface for Micao functions as a driver-like plug-in.
Xavier Levecq is cofounder and Jordi Andilla is an R&D engineer at Imagine Optic, 18 Rue Charles de Gaulle, Orsay, France; e-mail: [email protected]; biology.imagine-optic.com.
![FIGURE 2. Available microscope objectives have a wide variety of relative apertures (here plotted as 1/[f number], or #/f). The green box represents more than 90% of available objectives and corresponds to those that are compatible with Micao. FIGURE 2. Available microscope objectives have a wide variety of relative apertures (here plotted as 1/[f number], or #/f). The green box represents more than 90% of available objectives and corresponds to those that are compatible with Micao.](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2016/01/50482.png?auto=format,compress&fit=max&q=45&w=250&width=250)