BIOMEDICAL IMAGING: MIT researchers obtain 3-D images of living cells
Tomographic phase microscopy can provide detailed images of the activity inside a living cell, without the use of fluorescent markers or other contrast agents that could interfere with the results, according to Michael Feld, director of the George R. Harrison Spectroscopy Laboratory at the Massachusetts Institute of Technology (MIT; Cambridge, MA) and a professor of physics. Feld’s research group built imaging devices and interferometers and combined them with a standard CCD camera to develop an imaging technique similar to x-ray CT (computed-tomography) scans that enables scientists to create the first 3-D images of a living cell.
“Light scattering provides important information about the size distribution of substances inside the cell when considering subcellular surgery,” Feld said. “Once we have a tomographic image, we can see how cellular functions change. For the first time, we can study the functional activity of living cells in their native state.”
To create a 3-D image, the researchers combined 100 2-D images taken from different angles and obtained 3-D maps of the refractive index of the cell’s organelles in less than a second. An interferometer provided quantitative phase images from time-dependent interference patterns induced by the frequency shifting of a reference beam relative to the sample beam. A HeNe laser beam (633 nm) was divided into sample- and reference-arm paths by a beamsplitter. A galvanometer-mounted tilting mirror varied the sample’s angle of illumination. In the reference arm, the laser beam passed through two acousto-optic modulators that shifted the frequency by 1250 Hz. A second beamsplitter recombined the sample and reference laser beams, forming an interference pattern at the image plane. For each angle of illumination, a CMOS camera recorded four images at 500 frames. Phase images were calculated by applying phase-shifted interferometry with special software algorithms.
No cell preparation needed
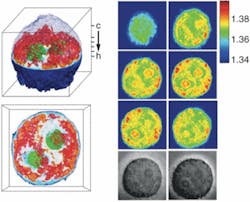
Feld’s team has used the quantitative, high-resolution technique to create 3-D images of cervical-cancer cells (see Fig. 1), showing internal cell structures, as well as the C. elegans worm and other organism types (see Fig. 2). Because the source of contrast is the refractive index of subcellular components, tomographic phase microscopy can be used to study live cells without any preparation. With other 3-D imaging techniques, the samples must be fixed with chemicals, frozen, stained with dyes, metallized, or otherwise processed to provide detailed structural information.“When you fix the cells, you can’t look at their movements, and when you add external contrast agents you can never be sure that you haven’t somehow interfered with normal cellular function,” Feld said.
The MIT researchers based their technique on the same concept used to create 3-D CT x-ray images of the human body, which are generated by combining a series of 2-D x-ray images taken as the x-ray source rotates around the object; the 3-D representations are reconstructed from multiple images taken from multiple directions.
The team’s image of a cervical-cancer cell revealed the cell nucleus, the nucleolus, and a number of smaller organelles in the cytoplasm. The researchers are currently in the process of better characterizing these organelles by combining the technique with fluorescence microscopy and other methodology. Current resolution is about 500 nm, but the team is working to increase this at least threefold.
“By improving the optics, we are confident that we can attain 150 nm or perhaps higher resolution,” Feld said. “We expect this new technique to serve as a complement to electron microscopy, which has a resolution of approximately 10 nm. We want to establish this as a useful technique and build instruments based on it.”
The research was conducted at MIT’s Laser Biomedical Research Center and funded by the National Institutes of Health and Hamamatsu Corporation.
Ilene Schneider | Freelance writer
Ilene Schneider is a freelance writer living in Irvine, CA.