OPTOELECTRONIC APPLICATIONS: BIOMEDICAL IMAGING - Multiphoton microscopy takes biologists into uncharted territory
One of the great advantages of optical microscopy is its ability to let scientists peek beneath the skin’s surface and study cellular processes at work. In recent years laser scanning confocal microscopy has established itself in the biological research community as a valuable tool for obtaining high-resolution images of live cells. But as biologists push to delve further into the inner workings of cellular infrastructures, the limitations of confocal microscopy begin to emerge-notably, the laser energy necessary to achieve optimum resolution can damage the very cells you want to image.1
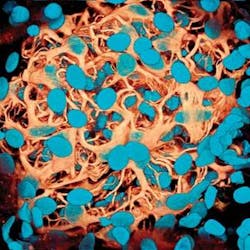
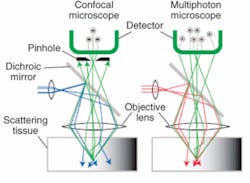
An alternative to confocal microscopy for biomedical imaging is multiphoton microscopy (MPM), which combines scanning microscopy with multiphoton fluorescence to create high-resolution, 3-D images of microscopic samples of living cells and tissues (see Fig. 1). Multiphoton microscopy is a nonlinear, fluorescence-based imaging method in which two or more photons from a long-wavelength, near-infrared pulsed laser excite fluorescent probes in only a single optical section without phototoxic side-effects (see Fig. 2).Although the instrumentation is still much more expensive than other optical microscopes-due in large part to the ultrafast laser sources required for two- and three-photon excitation that can make a single system cost more than $200,000- multiphoton microscopy offers certain key advantages, particularly for living cell and tissue studies.2 In addition to higher axial resolution and greater sample penetration, multiphoton microscopy improves the signal-to-noise ratio by eliminating fluorescence except at the focal point of the laser. It also can noninvasively image up to 500 µm below the tissue surface (compared to 20-30 µm with confocal), and the use of low-average-power lasers reduces sample photobleaching and phototoxicity and increases cell viability. Also, because many conventional fluorochromes used in multiphoton microscopy have very wide excitation spectra, a single wavelength can simultaneously excite a variety of these dyes in a specimen.
“From the life-sciences perspective there is a greater and greater desire to do in vivo imaging-particularly in animal imaging because it means you no longer have to sacrifice the animals,” says John Girkin, associate director of the Institute of Photonics, University of Strathclyde (Glasgow, Scotland). “Multiphoton microscopy gives you the ability to watch real-time in vivo imaging and observe the biology taking place right in front of you.” This makes it ideal for applications in immunology and histology, he notes, such as the study of cellular changes in cancer and Alzheimer’s and how these and other diseases progress over time.
Tweaking the optics
Although multiphoton excitation has been demonstrated with high-power continuous-wave argon and krypton lasers, the two main types of lasers currently used for multiphoton microscopy are ultrafast near-IR Ti:sapphire (700-1100 nm) and Nd:YLF (1047 nm) lasers. One reason these lasers are preferred is that, although they are more expensive, they do not require water-cooling and do not have excessive electrical-power demands. And given the Ti:sapphire laser’s tunability, which makes it more versatile than the single-wavelength Nd:YLF laser, it has become the laser of choice for most multiphoton-microscopy systems.
“The majority of MPM work is done around 800 nm, and the wavelength is often a compromise between not damaging your cell, getting a good image, and going deeply,” Girkin says.
In recent years, Carl-Zeiss and Leica have begun offering microscopes specifically designed for multiphoton applications. For example, Zeiss-the market leader in multiphoton-microscopy instrumentation since acquiring Cornell’s licensing of the original patents from Bio-Rad Laboratories in 2004 and then purchasing Bio-Rad’s laser-scanning-microscopy business that same year-offers the LSM (laser-scanning microscopy) line, which includes the LSM 510 NLO (nonlinear optics) and the LSM 510 Meta NLO. Both utilize a near-IR femtosecond laser that emits pulses with pulse energy of up to 170 kW and repetition rates from 76 to 90 MHz. One of the key advances in making systems more attractive for the life sciences is what the laser manufacturers have done to make the Ti:sapphire lasers more “biologist friendly,” according to Girkin.
“Five years ago if you wanted to do this kind of imaging, you had to buy a laser with 10 knobs on top,” Girkin says. “But the major laser manufacturers have invested in making the technology much simpler. The biology is phenomenally complicated, so they have focused on making the physics and laser engineering as easy as possible. With the easier-to-use Ti:sapphire lasers that are becoming available, you can more easily tune them. This is another reason the field has advanced over the last few years-biologists used to be more scared of adjusting the laser.”
At this point, however, most of the lab-based microscopes used for multiphoton microscopy are still actually modified scanning-laser confocal systems augmented with an ultrafast laser, according to Watt Webb, professor of applied physics and former director of the Developmental Resource for Biophysical Imaging and Opto-electronics at Cornell University (Ithaca, NY). Among other things, Webb is credited with coinventing multiphoton microscopy (along with Winfried Denk, currently director of the Department of Biomedical Optics at Max Planck Institute for Medical Research, Heidelberg, Germany).3
“Much of the most interesting research in MPM has come from home-built instruments,” Webb said. “The commercial instruments are not very flexible so you want to mess with them, and those who are capable of this are doing it because they have biological and biomedical imaging problems to solve.”
In fact, the very thing that makes multiphoton microscopy attractive to biologists-the ability to image deeper into tissue-creates a whole new set of issues related to resolution and contrast. The optical properties of the samples themselves start to degrade the image. Thus, as the resolution decreases so does the signal, forcing the laser power to be increased-and, in turn, running the risk of “cooking” the tissue. As a result, several research groups are pursuing optical techniques to enhance the resolution capabilities of MPM in deeper tissue without overdoing the power levels.
One approach involves “stealing” the adaptive optics technologies originally developed for astronomy. At the University of Strathclyde, for example, Girkin and colleagues have adopted a deformable-mirror approach that uses a beam-scanning system because sample scanning is not practical for most biological applications. While astronomers measure the wavefront aberration and then apply a correction to overcome the wavefront distortion, Girkin’s group uses algorithms that optimize a range of merit parameters, including image brightness and contrast. While the corrections may not return the system to the ultimate performance level, Girkin says they do provide a practical solution for the user; for example, they have been able to show that, for a number of rat-brain sections, using a single correction shape for a given depth retains around 80% of the ultimate performance of the system.
“What we are trying to do with the adaptive optics is exactly like the astronomers: remove the distortions,” Girkin says. “Normally these things are scanned and you do it point by point, very rapidly, with a different shape for each point on the image. But this would take a long time because you change the shape of the mirror each time. So we’re looking at using one shape across a range of the cross section of, say, brain tissue. It may not be the perfect physics solution, but it is a very practical, pragmatic approach that is relatively easy to use.” Girkin’s group is also looking at using a spatial light modulator to overcome the fact that the adaptive optics can only correct for a certain number of aberrations; while it is currently a slow process, they are thus able to do small corrections with the adaptive-optics mirror and then larger corrections with the modulator.
Going even deeper
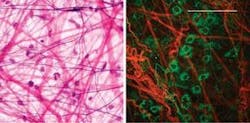
Webb’s group at Cornell believes it will one day be possible to take multiphoton-microscopy technology even deeper inside the body via endoscopes to enable clinical diagnoses of tissues not readily accessible by other methods. They have already demonstrated the ability to do MPM through an endoscope using gradient-index lenses, thus enabling minimally invasive, subcellular resolution several millimeters inside an intact anima.4 More recently, the group has been collaborating with Cornell’s Weill Medical College in New York City to build and test an endoscope developed specifically to work with MPM in medicine, according to Webb. They are also developing a related atlas of images that will enable pathologists to compare traditional tissue biopsies with the images that can be obtained using multiphoton microscopy (see Fig. 3).“If we’re going to use a new imaging technique like this (in medicine), we need to convince the pathologists because they’ve been looking at whether tissue is healthy or not for centuries,” he said. “We need to build an atlas of the MPM images versus the pathologists’ images to document the capabilities of MPM. That is the first step. Then we also need to develop the endoscopes; what is critical there is that we need very bright femtosecond pulses down the fiber.” They are currently working with a Ti:sapphire laser and a solid-silica-core fiber surrounded by an air-filled honeycomb structure made of very thin glass, which makes its refractive index similar to air.
Ultimately, Girkin believes that for multiphoton microscopy to find its way into a true clinical environment, the Ti:sapphire laser would likely have to be replaced by another type of laser that might not offer the same flexibility and tunability but would be more cost-effective. His group at Strathclyde has been experimenting with other diode-pumped solid-state lasers for this purpose, he added.
REFERENCES
1. M. Eisenstein, Nature 443, 1017 (2006).
2. L.A. Pray, The Scientist 18 (20) 40 (2004).
3. W. Denk, J.H. Strickler, W.W. Webb, Science 248 (4951) 73 (1990).
4. M.J. Levene, D.A. Dombeck, K.A. Kasischke, R.P. Molloy, W.W. Webb, J Neurophysiol 91, 1908 (2004).
About the Author
Kathy Kincade
Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.