From more-accurate diagnoses to less-traumatic surgical procedures, minimally invasive medicine has been changing the course of disease assessment and patient care for more than two decades. A critical component of this revolution has been the endoscope—an instrument that allows physicians to see and navigate inside the body through natural openings such as the ear or throat or through small incisions in the skin.
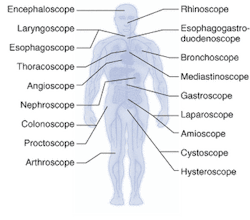
Endoscopes have been around for more than 100 years, although they were not used regularly in clinical practice until the early 1900s. The first endoscopes were rigid in nature; semiflexible endoscopes were developed in the 1930s, followed by truly flexible fiberoptic-based scopes in the late 1950s.1 The first clinical applications for endoscopes were in gastronomy and arthroscopy; today, site- and organ-specific endoscopes are used all over the body for dozens of surgical and diagnostic applications (see Fig. 1).
null
In fact, advances in optics, fibers, charge-coupled devices, microelectronics, and software are allowing doctors to access and image parts of the body that were previously difficult to get to, even endoscopically. Once there, these same scopes are enabling various surgical procedures—such as removing uterine fibroids, restoring vocal cords, and performing gastric bypasses—and critical diagnostic information to be collected in real time.
Smaller is better
The most significant recent advances in endoscopic technologies have involved miniaturization, image quality, in vivo imaging, and image analysis. Researchers have been working for more than a decade to apply endoscopy to the mammary ductal system, for example, where most benign and malignant breast lesions are thought to begin.2 Several groups have demonstrated the feasibility of mammary ductoscopy, but these efforts have been hampered by the inability to provide a small enough scope with acceptable optics and a working channel for insufflation and biopsy of the ducts.
The development of smaller, more-flexible scopes is also opening the door to new surgical and diagnostic applications in the gastrointestinal (GI) tract, sinus cavity, and brain. Because traditional endoscopic and radiology techniques are limited to macro-resolution imaging, conventional tissue biopsies are still required to detect dysplasia of the cells. But in the last decade, several techniques for in vivo imaging of tissue for real-time disease assessment have been developed, including optical coherence tomography (OCT), light-induced fluorescence endoscopy, and trimodal spectroscopy, all of which can detect and make indirect use of the structural and biochemical changes in cells associated with cancer and other diseases.
Researchers at the Endoscope Research Laboratory at Case Western Reserve University (Cleveland, OH), for example, are studying the use of endoscopic OCT to detect precancerous lesions in the GI tract. According to Andrew Rollins, assistant professor, biomedical engineering and medicine, OCT offers several advantages over existing diagnostic methods such as endoscopic ultrasound.3 "Our system has imaging resolution between endoscopic ultrasound and confocal microendoscopy," Rollins said. "We are not resolving individual cells, but we do achieve 10 times better resolution than endoscopic ultrasound."
null
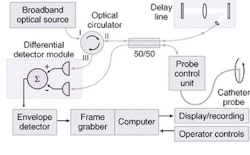
Rollins and his colleagues have been working with Olympus (Tokyo, Japan) on a prototype that combines a rapid-scanning OCT interferometer, catheter probes, and real-time data-capture-and-display hardware and software (see Fig. 2). The system has been tested in the esophagus, stomach, duodenum, ileum, colon, and rectum and is now undergoing clinical testing to assess its feasibility as a screening tool in Barrett's esophagus. "If we can find dysplasia in the GI tract earlier, then it is more treatable," Rollins said. "This is a method that could provide real-time histology information, which would really benefit procedure time and diagnostic accuracy."
Confocal microendoscopy
Other research groups are working to combine confocal microscopy with endoscopes to directly visualize the same structural changes in cells that pathologists use when assessing conventional tissue biopsies. By extending standard confocal microscopy into the body and using either fluorescence- or reflectance-based imaging, physicians and pathologists gain the ability to look at the cellular structure of tissue up to a couple of hundred microns deep.
"Confocal microendoscopy is different from other optical-biopsy techniques in that it allows you to get an image at various depths of the tissue," said Jaques Van Dam, professor and clinical chief, division of gastroenterology and hepatology at Stanford University (Stanford, CA). "It is the least tested method but has the greatest potential, especially in the bladder, cervix, mouth, esophagus, colon, sinus, and brain."
Van Dam is not alone in his enthusiasm for confocal microendoscopy. Because most cancers originate in the epithelium that lines the internal surface of organs, the ability to see abnormal tissue structures in the epithelium with micron resolution should allow earlier detection and more accurate location of lesions. While confocal microscopes can achieve optical sectioning and have been adapted for in vivo imaging of the skin, cornea, teeth, and cervix, confocal microendoscopy has the added benefit of being able to go deeper into the body.
"If you can actually see the pathology of the cells rather than infer them through changes in the optical properties, that makes the most sense," said Calum MacAulay, head of cancer imaging at the British Columbia Cancer Research Center (Vancouver) and chief scientist for DigiOpt (Bellingham, WA), a startup that is developing a digital confocal microendoscope. "And not only can you see it, but you can measure it and do quantitative analysis of it."
Several endoscopic systems have been developed for imaging deeper within the body using vertical-cavity surface-emitting laser arrays, micro-machined scan mirrors, single optical fibers, and fiberoptic bundles with gradient index lenses. Researchers at the University of Washington Human Interface Technology (HIT) Laboratory (Seattle, WA) have developed a flexible scanning single-fiber endoscope that provides high resolution and a wide field of view by placing a microscanner on the tip.4 According to Eric Seibel, assistant professor in the department of mechanical engineering and principal researcher, the system includes a prototype micro-optical scanner designed to overcome many of the issues related to fabricating small-diameter optomechanical scanners.
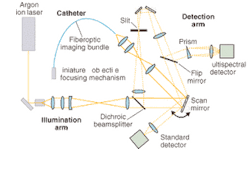
At the University of Arizona Optical Sciences Center (Tucson, AZ) researchers are taking a slightly different approach. Under the direction of Arthur Gmitro, professor of radiology and optical sciences, they have developed a microendoscopic imaging system based on a flexible fiberoptic catheter coupled to a slit-scan confocal microscope.5 The catheter, which comprises a fiberoptic imaging bundle containing 30,000 optical elements, a miniature objective, and a miniature focusing mechanism, is designed to fit through the therapeutic end of an existing endoscope (see Fig. 3).
null
So far, Gmitro and his colleagues have experimented with using the instrument in two ways—catheter-based imaging in the standard endoscopy arena and bare fiber through a biopsy needle. In the first approach, they have focused on imaging in the GI tract, the ovaries, and other peritoneal tissues via a small incision in the abdomen (see Fig. 4). In the bare-fiber approach, they have started some work in brain biopsies. In both cases, the group has opted to take a fluorescence-rather than reflectance-based approach.
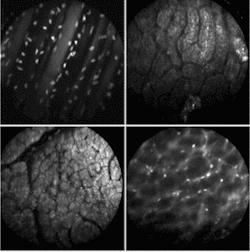
FIGURE 4. Multispectral fluorescence confocal microendoscopy offers the ability to do disease-specific imaging in real time using different phorofluors. Acridine orange (AO) is used extensively in multispectral experiments because of its dual emission peaks. Shown above are: in vivo image of the abdominal wall of a mouse, topical staining with SYTO 16 (upper left); in vivo image of surface of rat kidney, topical staining with AO (upper right); in vivo image of surface of rat liver, topical staining with AO (lower left); in vivo image of the retroperitoneum of a mouse, topical staining with AO (lower right),
"Fluorescence has some unique capabilities in designing phorofluors that are disease-specific and enable full-spectral distribution," Gmitro said. "So we could potentially use multiple dyes for multispectral imaging."
On the commercial front, Olympus is reportedly using MEMS technology to attach optical actuators to the distal end of its single-fiber microendoscopes, while Pentax (Tokyo, Japan) is partnering with Optiscan (Melbourne, Australia) to develop a high-magnification flexible endoscope. Optiscan already sells a confocal microendoscopy system for dermatology and is adapting the system for pathology.
Other startups in this emerging field include DigiOpt, which is collaborating with the British Columbia Cancer Research Center (BCCRC, Vancouver) on preclinical trials of its fiber-bundle-based microendoscopy system.6 Initially the goal is to assess its use for in vivo imaging of cells and tissue structure in the lung and esophagus; eventually the company plans to target biopsy guidance, midsurgery optical biopsy, and virtual biopsy in various parts of the body. The DigiOpt system uses an array of tiny micromirrors to control passage of illuminating and signal light through the fiber bundle. The fiber bundle can be selectively illuminated, and the reflected light or tissue autofluorescence can be filtered out on a fiber-by-fiber basis using the mirror array, which was developed by Texas Instruments (Dallas, TX).
"Other groups are using either reflectance or fluorescence, or, in some cases, both," MacAulay said. "Our objective is to do this without contrast agents."
Operating room of the future
Looking ahead, other endoscopic advances being pursued include the ability to make endoscopes "wireless" by using light-emitting diodes instead of xenon light sources, new optical molding techniques for the lenses, new fiberoptic materials, infrared laparoscopy, and virtual endoscopy. "With all these advances (including 3-D and advanced image processing), it comes down to one thing: when surgeons are doing an open procedure, they have tactile feedback," Leiner said. "With endoscopic procedures, they lose that. These new devices are intended to replace touch with technology."
REFERENCES
- D. Leiner, Endoscope Tutorial, Lighthouse Imaging, www.lighthouseoptics.com.
- J. R. Dietz, The Breast Journal 6(3) 161 (2000).
- A. M. Rollins, and R. Ung-arunyawee, Opt. Lett. 24(19) (Oct. 1, 1999).
- E. J. Seibel, and Q. Y. J. Smithwick, Lasers in Surgery and Med. 30,177 (2002).
- A. R. Rouse, A. Kano, and A.F. Gmitro, Proc. SPIE 4613 (2002).
- Digital Light Confocal MicroEndoscopy, confidential white paper, Digital Optical Imaging and Cancer Imaging Dept., British Columbia Cancer Res. Center, Vancouver, BC (September 2002).