Biomedical Imaging: MEMS scanners enable in vivo 3-D OCT
KARTHIK KUMAR, THOMAS E. MILNER, AND JOHN X. J. ZHANG
While nonoptical medical imaging techniques such as computed tomography, magnetic-resonance imaging, and ultrasound can be very useful in guiding surgical procedures, they lack the spatial resolution required for revealing tissue microarchitecture, which can be important in time-critical applications, such as staging of tumors.1 In contrast, optical imaging modalities such as confocal, two-photon, and fluorescence microscopy, and optical coherence tomography (OCT) provide depth-resolved imaging even in turbid media, with cellular-level detail.
In particular, OCT offers imaging depths of several millimeters through human tissue with micrometer resolution in all three dimensions and can extract additional properties of the sample (such as tissue birefringence, through polarization sensitive OCT); in combination with other modalities (such as ultrasound in Doppler OCT), it can provide the clinician with an enhanced 3-D view of tissue morphology.2, 3 But to enable imaging in small and sensitive human organs, optical diagnostic equipment—such as 3-D OCT probes—for clinical implementation require miniaturization of distal scanning mechanisms and advances in catheter design.
Mechanisms of beam deflection
The lack of miniaturization in conventional endoscopes limits their imaging capability in small and sensitive organs. Side-imaging OCT probes, which are relatively easy to assemble, have been extensively studied for imaging in tubular organs such as the gastrointestinal tract and esophagus; probe diameters of 0.4 to 0.5 mm have been achieved for this type. The difficulties in assembling forward-imaging probes are reflected in the smaller number of studies devoted to them.
The main problem relates to the mechanism of beam deflection across the sample. Previous studies have used fiber scanning with the fiber mounted on piezoelectric cantilevers or electromechanical actuators, objective-scanning using tuning forks, moving entire fiber gradient-index (GRIN) lens assemblies, or rotating an angle-polished GRIN lens for distal tip beam deflection.4, 5, 6 These methods typically suffer from one or more drawbacks, including aberration-limited field of view, slow scan rates, and nonlinear spatial scanning, which complicate image reconstruction. Fiber-optic bundles comprising closely packed single-mode fibers have also been used for beam deflection by selecting, at the proximal end, the fiber through which illumination is directed, and scanning through all the fibers sequentially.7 This method achieves significant miniaturization but suffers from lower spatial resolution due to crosstalk between adjacent fibers.
Miniaturization with MEMS
Microelectromechanical systems (MEMS) offer the unique capability of compact packaging of optical elements with microscale actuators, making possible the translation of advanced OCT techniques from benchtop prototypes in research laboratories to endoscopes in clinics. MEMS mirrors that angularly deflect collimated light from the back focal plane of a scan lens, converting it into a linear scan of a focused beam spot, are the central element in single-fiber forward-imaging endoscopes. Micromirrors using a thermal bimorph actuation scheme have been used for time-domain OCT endoscopes.8
Micromirrors that provide rotation about a pivot point at the center of the mirror are easier to integrate into optical scan systems and reduce optical aberrations. In particular, two-axis rotation about the mirror center enables 3-D volumetric image reconstruction through OCT without the need for relay systems between two 1-D mirrors.9 Micromirrors driven by electrostatic vertical comb drives combine this feature with the advantages of high rotation torque, scanning angles, resonant frequencies (or settling times), and low mirror surface roughness and dynamic mirror deformation. The broader applications of MEMS scanning devices also include adaptive aberration correction, dynamic beam focus control, and high-speed swept-wavelength laser sources. 10, 11, 12
Micromirror-based 3-D swept-source OCT
Spectral-domain techniques in OCT provide significant signal-to-noise-ratio advantages over time-domain OCT.13 Swept-source OCT (SS-OCT), in particular, has potential for sensitive, real-time, high-resolution imaging because of continual improvements in laser spectral range, line width, and scan time. Our fiber-based SS-OCT system uses a high-speed laser that scans a 110 nm wavelength range centered at 1310 nm, and is capable of performing 20,000 wavelength scans per second. Steinheil triplet lenses (with a numerical aperture of 0.6 and a focal length of 7.9 mm) focus light from the sample and reference arms onto the stationary mirror and sample, respectively. An inverse Fourier transform of the detected signal provides a map of the sample reflectivity as a function of depth at a specific lateral position. The lateral position is varied in a 2-D raster pattern using the MEMS scanning micromirror to develop a 3-D image of the sample volume. The system configuration provides high sensitivity, ease of alignment, portability, and maneuverability.14
We used an electrostatic staggered vertical comb-driven silicon micromirror of rectangular shape 500 × 700 μm in size to reflect a 0.5-mm-diameter collimated beam at a 45° angle.15, 16 The mirror comprises 30-μm-thick silicon coated with a metal film to provide uniform broadband reflectivity of approximately 90%, and is suspended by microscale torsion springs in a two-axis gimbal structure to provide rotation about the mirror center (see Fig. 1). Comb banks connected to the mirror structure are created along each set of torsion springs, and are interdigitated with a set of stationary combs in a separate layer of silicon.
Applying a voltage to the stationary layer creates an electrostatic force that draws the movable comb banks toward the stationary layer, causing the micromirror to rotate. Careful design ensures electrical isolation between the two silicon layers, as well as between orthogonal comb banks in the same silicon layer. Our micromirrors exhibit resonance on the inner and outer axes at 2.3 kHz and 390 Hz respectively, and provide ±9° of optical deflection on both axes for applied voltages of 110 VDC at low frequencies, or 20 VAC at resonance. Because of the highly capacitive nature of the electrostatic actuators, the MEMS actuation requires very little power and draws miniscule currents—usually 1 to 10 nA for nonresonant operation, or 2.4 μA in resonant mode.
Results, both 2-D and 3-D
We acquired reflectivity data over a 2 × 1 × 4 mm3 sample volume for rigid structures, in vitro biological samples, and in vivo human epidermis to demonstrate the capabilities of our MEMS micromirror-based SS-OCT instrument. The wavelength-swept laser enables MEMS OCT imaging with 8.6 μm axial resolution; the triplet lenses focused the 0.5-mm-diameter collimated scanning beam to provide lateral resolution of 12.5 μm. The system acquisition rate is greater than 10 million pixels per second, allowing an entire volume scan to be completed in approximately 15 seconds—an order-of-magnitude improvement over traditional time-domain systems.
One of our packaged silicon micromirrors, without metal coating, served as the first test sample (see Fig. 2). The information present in the entire 3-D volume is represented in an en face view by integrated reflectivity along the axial direction. Microscale features, such as the torsion springs and comb banks, are clearly visible in the en face and slice images across the 3-D volume.
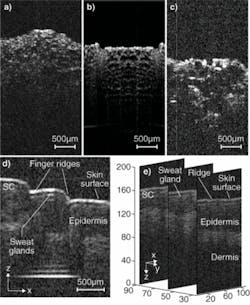
We acquired 2-D tomographic images of in vitro biological samples at 40 frames per second (with 512 transverse pixels per image) by operating the micromirror on only one rotation axis. Images of pickle slices were taken using the micromirror and, for comparison, a traditional galvanometer; an onion slice was also imaged using the micromirror (see Fig. 3a-c). We also obtained real-time 3-D images of in vivo human finger skin using the MEMS OCT instrument; microscale tissue architecture including skin surface, finger ridges, sweat glands, stratum corneum, epidermis, and dermis are clearly identified in the tomographic slices (see Fig. 3d-e).
Considerations and future developments
The lateral and axial resolutions of the instrument are governed independently by the scanning optics and the swept-wavelength laser, respectively. The diameter of the micromirror limits the maximum beam diameter incident on the scan lens, limiting numerical aperture and thus the field of view. The number of resolvable points in the image can be improved by increasing the product of micromirror diameter and scan angle. The choice of scan lens determines the lateral field of view and resolution, and depends on the requirements of the specific imaging application.
Miniaturization of scanning optics with the use of fiber-fused GRIN collimators, stationary microprisms, and other MEMS packaging techniques, such as flip-chip bonding for electronics integration, will enable fast diagnostic imaging in urinary/reproductive tract and pulmonary examinations and for gastroenterology. Real-time in vivo 3-D visualization at micrometer resolution can aid in minimally invasive disease detection, image-guided biopsy, and photodynamic therapy.
ACKNOWLEDGMENTS
The authors wish to acknowledge important contributions by Jonathan C. Condit and Kazunori Hoshino, Biomedical Engineering, University of Texas at Austin, and Nate J. Kemp and Austin McElroy of Volcano Corp., San Diego, CA. Financial support of this research by the Wallace H. Coulter Foundation Early Career Award, Veterans Administration, and National Institutes of Health grant R01 EY016462 is gratefully acknowledged. The micromirrors were fabricated at Stanford Nanofabrication Facility and UT-Austin Microelectronics Research Center, both supported by National Science Foundation under the National Nanotechnology Infrastructure Network.
REFERENCES
1. Z. Yaqoob et al., J. Biomed. Opt. 11, 063001 (2006).
2. J.F. de Boer, T.E. Milner, J. Biomed. Opt. 7, 359 (2002).
3. Z. Chen et al., Opt. Lett. 22, 64 (1997).
4. S.A. Boppart et al., Opt. Lett. 22, 1618 (1997).
5. A.L. Polglase et al., Gastrointest. Endosc. 62, 686 (2005).
6. J. Wu et al., Opt. Lett. 31, 1265 (2006).
7. T.Q. Xie et al., Opt. Lett. 30, 1803 (2005).
8. Y.T. Pan et al., Opt. Lett. 26, 1966 (2001).
9. Z. Hu, A.M. Rollins, Opt. Express 13, 6407 (2005).
10. N. Doble, D.R. Williams, IEEE J. Sel. Top. Quant. Elect. 10, 629 (2004).
11. V.X.D. Yang et al., Opt. Lett. 31, 1262 (2006).
12. Q. Chen et al., IEEE Photo. Tech. Lett. 16, 1438 (2004).
13. M. Choma et al., Opt. Express 11, 2183 (2003).
14. Y. Yasuno et al., Opt. Express 13, 10652 (2005).
15. U. Krishnamoorthy et al., J. Micromech. Sys 12, 458 (2003).
16. K. Kumar et al., J. Opt. A: Pure Appl. Opt. 10, 044013 (2008).
Karthik Kumar is a graduate research assistant at the Department of Electrical and Computer Engineering, University of Texas, Austin, Texas; e-mail: [email protected]; Thomas E. Milner is a professor and John X. J. Zhang is an assistant professor in the Department of Biomedical Engineering and the Microelectronics Research Center, University of Texas, 1 University Station Austin, Texas 78712, Austin, Texas.