Alexander J. Marker III and Bruce D. Jennings
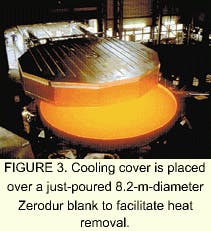
Any optical element greater than 30 cm (about 12 in.) in diameter or rectangular with the shortest dimension greater than 30 cm is considered large. Optical glass blanks in this size range find use as transmissive elements in telescopes, observation windows for wind tunnels and aircraft, reconnaissance camera lenses, radiation-shielding windows for nuclear reactors and hospitals, and lens systems for microlithography steppers (see Fig. 1). Other examples include large laser systems such as at the National Ignition Facility at Lawrence Livermore National Laboratory (Livermore, CA) and the Laser MegaJoule in France, both of which require meter-size glass slabs of neodymium-doped metaphosphate laser glass (see Fig. 2).Depending on the material—optical glass, laser glass, or glassy Zerodur—the manufacturing steps for large optical elements can change dramatically after the initial processing. Zerodur, for instance, is often used as a mirror substrate in astronomical telescope systems and requires additional processing to be transformed into a glass-ceramic (see Fig. 3).
Manufacturing maneuvers
The first step in manufacturing involves batching of the raw materials, a process in which the individual chemicals are properly measured and combined. These raw materials must be of high chemical purity, especially for optical and laser glasses, because common contaminants such as iron and transition metals can cause coloration, shifting of the ultraviolet (UV) cut-on characteristics of the glass, or an unwanted absorption at the laser wavelength of interest. Proper mixing of the constituents also is important because batch segregation can lead to fluctuations in the refractive index of the glass, which directly affects the optical homogeneity of the resulting glass blank.
The next step is melting, in which the initial batch reactions occur and the glass melt is formed. There is some preconditioning of the melt because convection currents cause a bit of blending in the liquid.
The refining stage, the first step in the homogenizing process, takes place at the highest temperature of the melting process, when the viscosity of the glass is low, thus allowing bubbles to rise to the surface. Because high-quality glasses must be free of bubbles, this degasification step is critical, and refining agents are often added to the batch. Their decomposition produces oxygen bubbles that absorb other dissolved gases in the melt and form large bubbles that rise to the surface faster.1
Next in the homogenizing stage is a continuous stirring process, which generally occurs at a lower temperature than the melting and refining stages and thoroughly distributes all components within the glass melt. This step is critical in eliminating striae and in producing uniform glass properties, for example, refractive index, over the entire glass blank. During this stage any fine oxygen bubbles are reabsorbed into the melt. The temperature of the melt is slowly reduced until the glass has a viscosity in the working range appropriate for the size blank being formed.
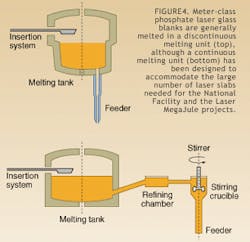
Forming takes place at temperatures such that the viscosity of the glass is in the working range defined by 103 to 108 poise. Melting can be performed in one of two ways—either in a continuous or a discontinuous melting process. In the continuous process, often referred to as tank melting, a batch is introduced into the input end of the melting unit, and conditioned glass is formed at the discharge end of the melter (see Fig. 4). For a fixed time, all the processes—melting, refining, stirring, and forming—occur at different positions in space. The amount of glass produced in a tank per day can range from a few to many tens of tons depending on the flow rate and the glass type.The discontinuous process, referred to as pot melting, is such that the process is fixed in space and is sequential in time. The batch material is introduced, the initial batch reactions occur, and a melt is formed. The temperature is then increased for the refining phase and lowered during the stirring phase to the forming temperature. Once the glass blank is formed, the process begins again. The amount of glass produced in a pot varies from several liters to a volume as large as 28 m3.
A coarse annealing process then lowers the formed glass to room temperature at a set rate such that the cold glass can be cut, ground, and polished for initial inspection of glass quality. This annealing is performed in a continuous fashion or in a batch lot with oven annealing. At this juncture, there is a difference between further processing of optical and laser glasses and glass-ceramics.
Processes diverge
Optical and laser glasses undergo a fine annealing process. The glass is placed in annealing ovens and taken to temperatures above the transformation temperature, the goal being to remove residual thermal stresses resulting from the forming process. The glass is then cooled very slowly, typically a few tenths of a degree per hour, to room temperature. The resulting finely annealed glass has very low residual stress birefringence.
For the glass-ceramic Zerodur, the glassy precursor is placed in special annealing ovens. The temperature is then increased to the nucleation temperature to form the nuclei on which crystals grow. The temperature is then further increased to the growth region where the crystal now grows in size. The glass-ceramic then goes directly into a fine annealing cycle in which the material is typically cooled at 6°C/h to room temperature.
Meter-class optical glass blanks are manufactured using both continuous and discontinuous melting units. The choice of the manufacturing technique is determined by the viscosity curve of the glass, the batch composition, and the interaction of the batch materials with refractory materials.2 Meter-class phosphate laser glass blanks are generally melted in a discontinuous melting unit. Recently, however, due to the large number of laser slabs needed for the National Ignition Facility and the Laser MegaJoule projects, a continuous melting unit has been designed for the manufacture of high-optical-quality phosphate glass.3
Zerodur blanks up to meter class are generally produced with a continuous melting process. However, for large blanks of the 2- to 8-m class, a discontinuous melting technique is required to obtain the flows needed to produce very large castings.4 After the fine annealing stage, the glasses or glass-ceramics undergo a final inspection and are prepared for shipment to the customer.
REFERENCES
- H. G. Pfaender, Schott Guide to Glass, 2nd ed., Chapman and Hall, London, England (1996).
- Geometrical Optics, Proc. SPIE 531, 2 (1985).
- J. H. Campbell et al., J. Non-Crystalline Solids, 263/264, 342 (2000).
- R. Hentschel et al., Conventional Production of Zerodur in Low Thermal Expansion Glass Ceramics, Hans Bach, ed., Springer-Verlag, Berlin (1995).
ALEXANDER J. MARKER III is the director of R&D and BRUCE D. JENNINGS is president and CEO of Schott Glass Technologies Inc., Duryea, PA 18642-2036; e-mail: [email protected]; [email protected].