A specialized adaptive-optics (AO) system created and demoed at Purdue University (West Lafayette, IN) takes high-resolution time-lapse images of functioning brain cells, and thus could be used to better understand how the brain works.1 The system is capable of revealing changing details of biological processes in cells over a larger field of view than otherwise possible, allowing high throughput essential for the study of brain activity.
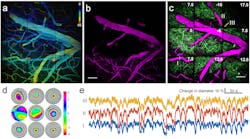
The prototype multi-pupil AO system includes 9 deformable mirrors and a prism array containing 9 faceted segments. Each segment produces its own image corresponding to a different part of a microscope's field of view. The approach improves the area of AO correction by 9 over a single-deformable-mirror approach; the field of view of the new system is 450 × 450 μm2. The system images voxels at rates up to 32 MHz, limited by the laser-scanning hardware.
"We are looking at huge numbers of neurons, so the number of data points you can measure per second is 20 million, 30 million," says Meng Cui, an assistant professor in Purdue University’s School of Electrical and Computer Engineering and the Department of Biological Sciences. "High throughput is very important because you want to measure these numerous neurons simultaneously at very high speed and also at high spatial resolution."
The researchers used the system to image brain cells called microglia; signaling processes of neurons involving calcium; vasculature in the brain; and dendritic spines, structures in neurons critical to learning and communication between brain cells.
"The microglial cells are important for maintaining brain health and recovering from strokes," says Cui. "High-resolution in-vivo imaging of dendritic spines is also of great importance in neuroscience. And calcium imaging has been widely used in neuroscience for in-vivo large-scale recording of neuronal network activity, which demands both high speed and excellent image quality."
The system was used to perform time-lapse imaging to study changes in functioning brain cells, in research with laboratory mice.
The imaging area could be further raised in the future by increasing the size of the prism array to as many as 36 segments.
REFERENCE:
1. Jung-Hoon Park et al., Nature Methods (2017); doi:10.1038/nmeth.4290

John Wallace | Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.