Cell Biology/Cardiology: Ultra-stable micromanipulators enable nanoscale force measurement
The leading cause of death in the industrialized world, cardiovascular disease, often results from dysfunction within individual heart muscle cells called cardiomyocytes (see frontis). These cells represent the smallest fully functional model system of heart muscle that can be examined for ion regulation, force production, relaxation function, cell signaling, and gene expression.
Many important avenues of research involve the study of individual cardiomyocytes, and such studies often use optical microscopes to examine live cells extracted from rodents. For instance, microscope-based instrument platforms allow a range of multimodal experiments to investigate both auxotonic contractions (which involve myocyte shortening) and isometric forces (which are generated without physical shortening). The correct operation of these two forces in individual cells enables the heart to perform the four stages (I-IV) of the cardiac cycle (see Fig. 1).Next-generation instruments aim to help advance this type of research. For instance, the latest MyoStretcher system from IonOptix promises ease of use along with superior sensitivity in both force and motion measurements—enabled by a pair of XYZ micromanipulators that allow researchers to pick up single myocytes.
Measuring myocytes
In use, each micromanipulator holds a glass rod (typically 25 μm in diameter and 500 μm long), which is dipped into a droplet containing MyoTak, an adhesive mixture of extracellular matrix (ECM) proteins and polysaccharides. After letting this partially dry, the operator contacts each end of a myocyte that is lying on chamber glass under buffer. It takes about 10 s for the cell to adhere to the rods, after which time it can be lifted off the glass for experimentation.
Once a myocyte has been picked up, it can be physically or electrically stimulated and/or its operation quantified in various ways (with optional accessories). For example, the system's piezoelectric actuators can apply mechanical stretching force to the cell, or the cell can be stimulated by using patch clamp-type electrodes.
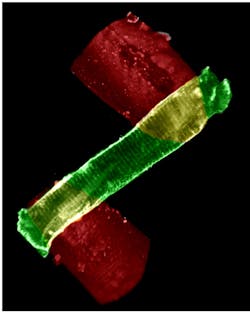
Individual myocytes generate contractile forces in the 10–1000 nN range that can be measured using a proprietary optical approach called OptiForce (see Fig. 1). Briefly, a cantilever is attached to the force transducer head. The position of a reflective surface at the distal end of the cantilever is determined interferometrically via backreflection of a fiber-coupled laser diode (at 1550 nm). Because the position of the myocyte can be measured to a tiny fraction of a wavelength, isometric action can be studied where the contraction is close to zero.
OptiForce enables observation of cardiomyocyte activity at the molecular level in two ways: by monitoring dynamic local changes in calcium ion activity or by measuring length changes of sarcomeres, the striations (dark and light bands) that result from the molecular assemblies involved in contraction. At rest, sarcomere spacing is around 1.8 μm. This instrument captures brightfield images under red light illumination in real time using a high-speed (250 Hz to 1 kHz) CCD camera. The system software performs fast Fourier transform (FFT) analysis of the imaged striations to obtain a very accurate average value of sarcomere length as the cell contracts or relaxes.
As widely used in microscope imaging of other metabolic processes, myocyte metabolic activity at the sub-cellular, molecular level can also be followed using calcium ion probes whose fluorescence characteristics are quantitatively dependent on the local concentration of calcium ions. This is accomplished by use of an integrated xenon lamp and an epifluorescence adapter, while using the camera in conjunction with a dichroic filter.
According to Dr. Joe Soughayer, applications scientist at IonOptix, "Our goal with the latest version of our instrument is to enable scientists to push the resolution and sensitivity of their measurements. In isometric experiments, a myocyte will exhibit total movement of only 1 μm or less. Ultimately, we want to reach the limit of the technique, which is set by the compliance of the MyoTak adhesive. We therefore had two key requirements for the micromanipulators. They needed to provide near-zero drift; sub-micron level drifts and/or play would make it impossible to accurately measure the tiny forces and movements our customers want to study. In addition, these are often complex and challenging experiments, so we wanted a system that featured simple, intuitive controls with no real learning curve, where users could focus on the science." The MyoStretcher incorporates a pair of these subsystems in right- and left-handed configurations, where the glass rod is mounted on the z-axis of each subsystem.
Choosing micromanipulators
There are numerous types of micromanipulators based on several different technologies, including hydraulics, micro-steppers, and piezoelectrics. IonOptix selected three-axis micromanipulators based on DC/servos—a customized OEM version of the MX 7630 from Siskiyou (see Fig. 2). The choice had to do with the advantages of DC/servos, which offer the requisite range of travel and resolution, whereas piezos don't have sufficient range of motion for the critical myocyte pick-up action. Moreover, a DC actuator can hold a position without drawing electrical power unlike steppers and piezos, for example. And a current draw inevitably creates unwanted heat even when stationary; that is, during the measurements. Thermal drift is always the enemy of precision positioning. So, DC actuators have the inherent potential for ultralow drift, even during extended experiments. In addition, some experiments may also include electrophysiology measurements, where any electrical magnetic interference noise (EMI) must be avoided because of the minute electrical signals involved.Among the DC/servo options, the MX7000 series demonstrated the lowest amount of play and drift compatible with MyoStretcher's target performance (measurement to 1 nN), while providing up to 20 mm of motion in each axis. A motorized actuator with anti-backlash gearhead (in each axis), driving a crossed roller-bearing highly linear stage, eliminates axial and lateral play, and a pre-loaded screw drive avoids the possibility of drift when stationary—that is, during a typical single myocyte experiment. Also, because the three-axis subsystems were originally developed for demanding electrophysiology (patch clamp) experiments, they are totally compatible with the limited access of a high-magnification optical microscope.
These micromanipulators also provide simple operation. Controllers have two preset speed settings: rapid (1.7 mm/s) and medium (300 μm/s). The third speed selector (slow) has a variable 330° potentiometer that enables continuous speed adjustment from 50 μm/s down to 2 μm/s. With the speed selector set at the slowest settings, consistent 0.2 μm moves are easily made by the simple bump of an axis button.
Several labs have already published studies enabled by 1 nN force sensitivity, and the technology promises to enable further discoveries that we hope will ultimately lead to reduction in heart disease and better treatment for those affected.
John Wingerd | Senior Mechanical Engineer, Siskiyou Corporation
John Wingerd is senior mechanical engineer with Siskiyou Corporation (Grants Pass, OR).

