Combined time-resolved microscopy with spectroscopy for materials science
Studying luminescence lifetime data is a very powerful analytical tool for spectroscopists and microscopists alike, as it provides insights into the excited state dynamics of molecules, complexes, nanoparticles, or semiconductors. The fluorescence or phosphorescence lifetime is an intrinsic characteristic of luminescent species. It indicates how long the species under consideration will remain in an electronically excited state before returning to the ground state. Each emitting species has a characteristic luminescence lifetime that can be influenced by its environment.
A series of spectroscopy and microscopy methods based on luminescence lifetime have been developed and allow obtaining information that would be otherwise not accessible through steady-state experiments. For example, fluorescence lifetime imaging (FLIM) is a very well established imaging method in life science where the lifetime information is combined with spatial localization in the sample, allowing investigating biochemical or physical processes. This combination of data can help detecting changes in the local environment such as pH, temperature, or ion concentration, identify molecular interactions or conformation changes via Förster Resonance Energy Transfer (FRET).
Time-resolved methods such as FLIM or Fluorescence (Lifetime) Correlation Spectroscopy (F(L)CS) are commonly used in biological studies, but similar methods such as Time-resolved Photoluminescence (TRPL) are also important in materials science for the characterization of key parameters like charge carrier dynamics and mobility in semiconductors.
The general methodology of TRPL can be expanded by performing imaging of charge carrier dynamics, which allows linking lifetime information with spatial location. This can be exploited to determine the effect and influence of carrier diffusion on the total lifetime measured in conjunction with intensity dependent photoluminescence measurements.
Using this method requires either a time-resolved microscope or coupling a time-resolved fluorescence spectrometer with a scanning microscope. A simple way to realize such a set-up is to use the FluoTime 300 fiber coupling sample mounting unit to interface the spectrometer with a microscope e.g., MicroTime 100. The full range of optical elements from the FluoTime 300 can be used along with the analytical tools of the EasyTau and FluoFit software packages to investigate light originating from the microscope focal volume.
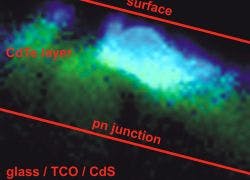
TRPL imaging is an exceptionally powerful tool for semiconductor analysis with respect to material and architectural substructures, spatial inhomogenities and process dependent morphology. Using TRPL imaging, charge carrier diffusion processes and the effect of localized inhomogenities and defect sites can be identified. With this multi-dimensional approach, a versatile and powerful methodology for the analysis of semiconductor materials can be achieved.
In order to acquire such a TRPL image, the photons have to be attributed to each different pixel, which is done by storing the absolute arrival times of the photons in addition to their relative arrival time with respect to the laser pulse. Line and frame marker signals from the scanner of a confocal microscope are additionally recorded in order to attribute the photon time stream to the corresponding pixels. Learn More.