Cytometry: Maximizing cytometer performance with optomechanical stabilization
Certain factors can make live cell analyzing and/or sorting particularly challenging. An approach developed by artificial insemination supplier ABS Global (DeForest, WI; a division of Genus plc) has enabled the company to maximize cytometer signal-to-noise to achieve cost-effective improvement of their product. The approach is predicated on high optomechanical stability, including the use of monolithic adjustable (flexure) optical mounts.
Modern cattle farmers make extensive use of artificial insemination with commercially acquired bull semen, and high optomechanical stabilization allows them to cost-effectively improve their blood lines and stock quality. Recently, there is growing interest in using bull sperm that is pre-selected to preferentially result in female calves for dairy farmers since they have much higher value than male calves. ABS’s cytometry enables the company to perform cost-effective sexing of bull sperm for this purpose.
Traditional approach
Flow cytometry is well established as a method for high-speed analyzing (counting) and sorting of cells and other small bio-entities. For example, it is widely used to perform blood cell counting and other tasks in medical clinical labs. In flow cytometry, the cells are diluted or concentrated as necessary and treated with some type of fluorescent dyes or markers often attached to an antibody, so that each fluorescent label only bonds to specific cell proteins and components. The cells are then forced to flow in single file droplets through a laser interaction zone, where they are irradiated by one or more focused laser beams. The fluorescence is spectrally filtered into several bands so that the type of each cell can be determined and counted. Where sorting is required, the standard method is to rely on the static charge picked up by the droplets and deflect them into one or more collection tubes based on their fluorescence signals.
This basic approach has been previously applied to sperm sorting. Like other mammals, the gender of a calf is determined by which sex chromosome is present in the parental sperm; the male chromosome Y results in male characteristics and X results in a female phenotype. Since the Y chromosome is essentially a significantly truncated version of the X chromosome, this means that male sperm contain less total DNA.
Before passing through the instrument, the sperm are treated with a benign fluorochrome (for example, Hoescht 33258) that binds to the minor groove of the double helix and produces blue fluorescence under ultraviolet (UV; 355 nm) laser excitation. In principle, the sex of the sperm can thereby be determined by the amount of fluorescence.
Special challenges
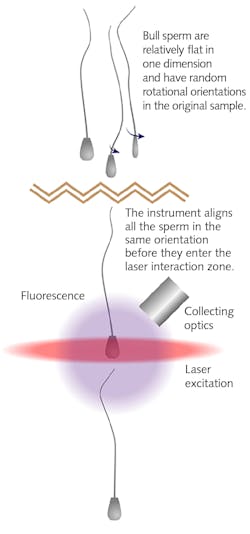
Practical implementation of this approach presents numerous challenges, and scientists at ABS Global set out to design, optimize and build a better production instrument for high-fidelity, high-volume sex determination of bovine sperm. This effort was led by Dr. David Appleyard, Principal Biosystem Engineer at ABS. Appleyard explains, “The difference in fluorescence intensity between male and female sperm is only 3.8% in cattle. Moreover, bull sperm do not have cylindrical symmetry; they are characterized by a flat head that contains the DNA and a long tail (see Fig. 1). This adds major potential anisotropy to the efficiency of fluorescence excitation and detection using a laser beam and a fixed photodetection axis. And unfortunately, commercial flow cytometers are configured to process cells that are close to spherical. So, errors associated with sperm orientation could be far greater than the 3.8% signal difference we are seeking.”
Unlike a blood count, in which the counted cells are simply discarded, the sperm cells are the final product at ABS—which they call Sexcel sexed genetics—and are quite valuable. So, they should not be harmed or damaged by the process. This makes minimizing sperm trauma, and therefore maintaining a high number of viable female sperm in every sample, a design imperative. And of course, high throughput (that is, high speed) is another critical performance parameter when the cytometry instrument is used as a manufacturing tool. (There are also some challenges in the chemical protocols since the fluorochrome uptake varies from breed to breed and even between individual bulls of the same breed.)
Low-noise cytometry
Appleyard explains that, “Starting from the ground up on this application gave us a lot of freedom with design concepts to achieve all these goals by using the latest technologies, and without being limited by existing flow cytometry paradigms. The first challenge was to deal with the sperm shape and potential signal anisotropy—without addressing this, the whole application is a non-starter.”
In traditional flow cytometers, the single-file flow is created by forcing the cells through a tiny sample tube located within the flow stream (formed by a larger sheath tube to create a stable hydrodynamic flow), which then passes through a tiny orifice. For these applications, it is usually essential to form droplets at this orifice—one cell per droplet. And, to preferentially orient the sperm, the sample tube terminates in a wedge shape to induce a ribbon-shaped flow.
However, ABS uses a completely different approach. Instead of electrostatically sorting the sperm, they decided to use high energy pulses from a Q-switched UV (355 nm) laser to terminally incapacitate the male sperm immediately downstream of the interaction zone of the low-power 355 nm analysis laser. Crucially, without the need for physically separating the sperm, there is no need to form droplets using a nozzle that can subject the sperm to shear forces and can also limit throughput speeds. Instead, ABS uses a microfluidic chip configured to benignly form the flow stream with preferential orientation of the sperm relative to the excitation and photodetection axes. The company also uses proprietary protocols to accommodate different bull-specific rates of uptake of the DNA fluorochrome (Hoescht 33258).
Minimizing signal noise is obviously very important when sorting sperm with their relatively low contrast metric (<4%). When combined with the potential errors from the angular sensing anisotropy, this impacts optomechanical design. According to Appleyard, “We use the typical elongated ellipse focus for the analysis laser where the long axis is perpendicular to the flow direction, which means that excitation efficiency is relatively insensitive to minor variations in the path of each sperm. However, maintaining precise alignment of the collection optics that bring the emission to the photodetector (avalanche photodiode, or APD) is absolutely critical. And the alignment and focus of the incapacitation laser is similarly critical, where we rely on just one high-energy focused pulse to terminate each male sperm.”At the same time, to make their product economically attractive, these flow cytometers have to feature high throughput—roughly 20,000 sperm/minute are passed through each of hundreds of separate instruments (see Fig. 2). So, some heat is inevitably generated in the confines of the instrument, which means thermal insensitivity of these alignments is also important. And, since these instruments are operated with a high duty cycle in a production environment, they also need to be insensitive to typical ambient vibrations, etc.
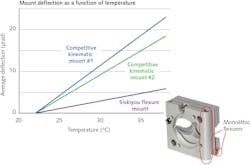
To meet these needs for optomechanical stability, critical optics for the termination beam and fluorescence collection such as mirrors/beamsplitters are aligned using novel monolithic dual-axis flexure mounts from Siskiyou Corporation (Grants Pass, OR). Unlike conventional flexures that are assembled from several components (brackets, leaf springs, etc.) which are often different metals, these lockable mounts are machined from a single block of metal: steel or aluminum (see Fig. 3). Consequently, the entire mount expands or contracts uniformly with temperature changes. Moreover, this monolithic construction also translates into improved heat transfer through the entire mount. Together, this makes their locked alignment relatively insensitive to ambient temperature changes. Plus, these mounts are available with a low-profile top-adjust option, simplifying their use in the confines inside a compact instrument such as a flow cytometer.Intensive quality control for success
There is an inevitable tradeoff between sorting efficiency and minimizing sperm trauma. This results in ABS shipping sex-selected sperm samples (called straws because of their package shape) that deliver conception rates at 90% relative to conventional sperm, while also offering a roughly 90% chance of each pregnancy resulting in a female calf. ABS customers report a very high level of satisfaction with their sex-selected products. In theory, the company could possibly achieve an even-higher bias—for example by running the samples through the instrument twice, but in practice, the additional handling starts to impact viability (conception rate). Internal quality control is an important part of maintaining the high sex ratio. After cryopreservation and prior to storage, two straws are randomly selected for destructive analysis from every batch from a particular bull—sex counting via conventional flow cytometry. Unless both testing samples indicate >85% sex bias, the entire batch is discarded.
ABS Global is applying a next-generation photonic instrument as a frontline production tool to improve profitability for dairy farmers. With a gestation period of about 283 days and a typically annual pregnancy cycle, the ability to sex-select for female calves with around 90% confidence delivers a substantial economic advantage to farmers, which well justifies the cost of the process.
ACKNOWLEDGEMENT
Sexcel is a registered trademark of ABS Global.
John Wingerd | Senior Mechanical Engineer, Siskiyou Corporation
John Wingerd is senior mechanical engineer with Siskiyou Corporation (Grants Pass, OR).