MEDICAL IMAGING: FEL reveals links between melanin structure and Parkinson’s
In a finding that may offer clues about Parkinson’s disease, a team of researchers from Duke University (Durham, NC), North Carolina State University (Raleigh, NC), and the Italian Institute of Biomedical Technologies (Segrate, Italy) used the free-electron laser (FEL) at Duke in conjunction with photoelectron-emission microscopy (PEM) and atomic-force microscopy (AFM) to image the structure of neuromelanin.1 Scientists know that neuromelanin comprises two pigments: eumelanin, found in black-haired people, and pheomelanin, found in redheads. But how those pigments are arranged structurally remained unknown, and that information could be of critical importance in better understanding disease.
In 2005 a Duke team that included some of the same scientists involved in the current study reported using the FEL to establish that pheomelanin activates oxygen, while eumelanin doesn’t. Oxygen activation is suspected to play a role in the neurogenic cascade of events behind Parkinson’s disease. In this latest study, the Duke researchers gained evidence that neuromelanins isolated from human brains have cores of oxygen-activating pheomelanin covered by a protective surface of eumelanin.
“This is the first piece of morphological data about how these pigments are constructed,” said study leader John Simon, professor of chemistry at Duke. “Of the two types of pigment in the brain, one is more antioxidant than the other and there are ideas that they are intermingled in either a random or structured way. We wanted to know what the oxidation potential looks like across the neuromelanin granules.”
Adding a dimension
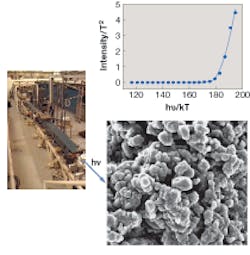
Simon and his colleagues used a PEM coupled to the tunable FEL to obtain wavelength-dependent UV (248 to 418 nm) images of isolated neuromelanin samples deposited on a silicon substrate (see figure). The AFM was added to enhance the 2-D PEM images with 3-D information. Using these devices in combination, the researchers were able to pinpoint the oxidation potentials of molecules coating the surfaces of neuromelanin granules. The team found that oxidation potentials of molecules at the surfaces approximated those found in black hair pigments in the 2005 study.
“That meant it was eumelanin, which is pretty antioxidant,” Simon said.
Neuromelanin granules begin appearing in human brains between ages 3 and 5 and their concentrations increase steadily thereafter. However, neuromelanin levels drop precipitously in the brains of Parkinson’s patients, who also experience a death of brain cells that are darkly pigmented and an increase in brain tissue concentrations of the metal iron. The Duke FEL study was designed to determine how neuromelanin contributes to this degradation and to understand what the pigment is and how it changes over the course of a patient’s life. Scientists have hypothesized that brain cells synthesize neuromelanin to serve as a defense mechanism against high oxidation stress and that neuromelanin’s layered granular structure could help protect brain cells from damage.
“What you see in Parkinson’s brain is about a third of the amount of pigment found in a normal brain,” Simon said. “So if it is metabolism and loss of pigment that contributes to disease, whatever causes the degradation produces material that contributes to the oxidation. The question is what is cause and what is effect.”
While the FEL may not be the most practical in terms of size and mobility, it is actually the best laser to use for studies like these because of its extreme tunability and beam characterization, noted Simon. He and his colleagues are currently doing some additional experiments with a frequency-doubled argon-ion laser, but he says that the ionization potential of human pigments requires an easily tunable laser because different pigments have different potentials.
“Could you take a conventional UV laser and do this? Yes. Could you do it in half an hour? No,” Simon said. “With the FEL, in 30 minutes it is easy to go from 220 to 400 nm, and all the parameters hold constant, the energy is known, the beam is well-characterized, and the spot stays in the same place. The FEL is the perfect choice for this application and an ideal source for tuning through the entire UV range.”
Simon and his team have since analyzed other properties of the granules and discovered new lipids that he says no one has seen before. They are also experimenting with polarization of the light to help determine if the surface molecules are aligned or random, which could provide additional information about the pigment structure and function.
REFERENCE
1. S. Ito, Proc. National Academy of Sciences 103(40) (Oct. 3, 2006).
About the Author
Kathy Kincade
Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.