JOHN GIRKIN
As multiphoton microscopy comes of age, it is perhaps a sensible time to assess how far the method has really made an impact in both the life sciences and the optics communities. The interactions among the end users—frequently with no optics or laser expertise—the optical and laser research community, and the commercial suppliers make an interesting case study in the way fields that use high-technology products develop. As the methodology and instrumentation reaches maturity, what lessons can be learned as technology crosses disciplines and where might the field go in the future?
The first multiphoton fluorescence imaging was undertaken using a colliding pulse dye laser, which was clearly not an instrument for routine use in a biology lab.1 However, the femtosecond Ti:sapphire laser came to the rescue, removing the need for messy dyes, and, crucially, improvements in laser diodes in the early 1990s led to the all-solid-state pump source for such lasers.
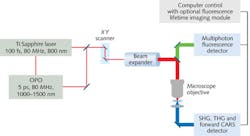
As the market for femtosecond sources grew, specifically for use by non-laser experts, commercial “hands-free” systems appeared to fill this market need and drive both the imaging and laser technology forward. Thus, a symbiotic relationship has developed between the technology users and suppliers2 (see Fig. 1).
Challenges to nonlinear microscopy
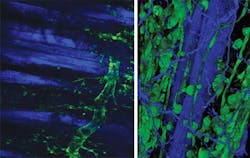
From a biological perspective the real advantage of nonlinear microscopy stems from the ability to image deeply with minimal perturbation to the sample. This then enables the researcher to look at the biology functioning in vivo. Thus the ability to image small animals is a strong driver, ideally for extended periods of time (see Fig. 2). Yet this motivation presents three challenges to the optical technology: beam delivery, pulse dispersion, and sample-induced aberrations.The first challenge is that the light now has to be delivered at depth potentially beneath the skin of the sample. Longer-working-length microscope objectives have been developed, but a recent technological advance has been the use of GRIN optics or directly shaping the ends of optical fibers, in particular photonic crystal fibers (PCFs).
Several university groups (including Harvard, Massachusetts Institute of Technology, Stanford, and Swinburne) have now built miniature beam-delivery systems.3–6 These have fallen into two classes: one in which the optics are used to simply relay the focus of the microscope objective into the sample using two simple lenses; the other in which a complete miniature optic system is built, removing the need for a conventional imaging system (see Fig 3).This later solution has the advantage of not needing a large conventional microscope in the system, but at present the optical quality is not as good as one would expect from objective-based systems. The area of developing high-quality miniature optics for such imaging systems is therefore in need of continued study. The use of high refractive index materials (greater than two) such as diamond or silicon carbide is potentially of interest, as these have all been used to produce small lenses by other groups.7
Having developed the beam delivery system a further problem has been created, that of pulse dispersion. The femtosecond pulses are now traveling through more glass and glasses with nonuniform refractive indices, which leads to pulse broadening mainly due to the group velocity dispersion within the materials. This can be partly overcome through the use of dispersion compensation, or pre-chirping, the laser pulse before it enters the optical system.
Commercial systems with this capability are now available (e.g., the Mai Tai DeepSee from Newport), although this does add a further level of complexity to the laser source even when fully automated. The use of a PCF with the correct dispersion systems is an area of active research, in particular for miniature instrumentation.
Linked with the miniature optics is the development of miniature scanners, thus enabling an entire miniature scanning system. This is a very active area of research for nonlinear imaging, but at present the main focus is via optical coherence tomography (OCT)-based devices with a wide range of novel designs, with either two-dimension scanning in a single device or dual scanners.
The use of miniature scanning systems based upon fluorescence, especially in samples with only certain cells expressing fluorescent protein, is complicated by the requirement to know exactly where the probe is within the subject. The images only provide a fluorescent image, and then only in the direction of view of the probe, making precise navigation difficult. This is a similar challenge to that encountered in the early days of minimally invasive surgery and the concept of including a miniature camera and reflected light for geographical guidance is one route to be explored, demanding very small but highly sensitive camera systems.
The final complication with deep in vivo imaging is that of sample-induced aberrations. The method that is being developed to overcome this is through the adoption of adaptive optics originally developed for astronomy to remove atmospheric turbulence from images.
The improvement in all forms of nonlinear imaging has now been demonstrated, but the best implementation route for each imaging challenge still has to be determined. At present most systems rely on a so-called “wavefront sensorless” approach using an optimization algorithm to determine the best mirror shape. Recently, groups at Durham University, Massachusetts Institute of Technology, and the University of California–Santa Cruz have started looking at a more conventional approach with a laser guidestar in some format and a wavefront sensor to determine the mirror correction that should be applied.8,9 The latter offers higher-speed imaging, but the optical system is more complex.
SPIM and longer wavelengths
A recent and interesting advance in nonlinear imaging is its use in single plane illumination microscopy (SPIM).10 Here, a sheet of light is projected through the sample and the resulting fluorescence viewed perpendicularly to the illumination source. This enables complete optical sections to be recorded at high speed, with the nonlinear excitation reducing unwanted scattering and photodamage.
The SPIM technique has already demonstrated its power in imaging live, semi-transparent samples such as zebrafish embryos and C. elegans, although as yet the full impact and potential of nonlinear SPIM remains to be shown. Because samples are frequently imaged for 24 or 48 hours as they develop, lowering the risk of unwanted effects caused by the illumination source is clearly crucial and thus it appears that the near-infrared light used in multiphoton imaging may have a role to play.
The other trend over the last few years has been the move to longer excitation wavelengths. Originally, conventional visible flurophores were used because these were readily available and well understood. However, as the advantages of nonlinear microscopy—and in this case the use of longer-wavelength light—became clear there was a move to longer wavelengths to further remove effects of light scattering, although care must be taken about water absorption.
This approach does present new challenges for the optical researcher, including a requirement for reliable femtosecond sources with repetition rates around 80 MHz and wavelengths from 1200–1800 nm. Many new materials have now been tried in this new laser “window of opportunity” and all provide interesting results, so it is not yet clear which is the best longer wavelength to use.
As the methodology of nonlinear imaging becomes established within biology, the obvious next direction is to move toward the clinic. Clearly some of the complications outlined here are still present and are challenges yet to be resolved, but working on patients leads to other challenges.
Toward clinical applications
Much of the work being undertaken on human imaging has been centered on the use of multiple imaging modalities because adding flurophores to patients is not always possible. Therefore, systems that can use natural flurophores within the body are preferred: for example, NADH, second harmonic signals from collagen, and then CARS for examining specific lipid structures.11
DermaInspect, a commercial instrument, is a nonlinear microscope with an imaging head that moves over the patient for inspecting skin lesions. To date, this has shown that different imaging modalities provide different information to the clinician, but the crucial step now is determining whether this instrument can improve diagnosis and subsequent treatment planning.
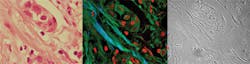
Building on the miniature endoscopes currently being developed for biological use, clinicians have started to look at multiphoton imaging for minimally invasive biopsies (see Fig. 4). At present the majority of this work is considering previously excised tissue, but the initial results are encouraging.With patients involved the systems must work every day, be robust to the external environment, and be easy to use by several different staff members. This is an opto-mechanical challenge that should not be underestimated and even with today’s “one-package” femtosecond lasers, the technology is not ready. Cost also needs to be driven from the instrumentation if it is to have widespread acceptance because in the current financial climate—even in the clinic—the cost-benefit of new instruments is a growing factor in purchasing decisions.
It is thus clear that nonlinear microscopy has developed greatly as it reaches its 21st birthday. It has reached a level of maturity within its home field of the biology laboratory, but to reach the larger and potentially very important clinical market significant work is still required. Clinicians, life scientists, physicists, and engineers all need to work together to bring out the potential that is clearly present in the young adult technology.
REFERENCES
1. W. Denk et al., Science, 248, 4951, 73–6 (1990).
2. J.M. Girkin, J. Phys. D: Appl. Phys., 36, 14, R250–R258; doi:10.1088/0022-3727/36/14/204 (2003).
3. R.P.J. Barretto et al., Nature Meth., 6, 7, 511–2; doi:10.1038/nmeth.1339 (2009).
4. D. Kim et al., “Optical biopsy in high-speed handheld miniaturized multifocal multiphoton microscopy,” Proc. SPIE, 5700, 14–22 (2005).
5. B.G. Saar, et al., Opt. Lett., 36, 13, 2396–2398 (2005).
6. H. Bao and M. Gu, Opt. Exp., 17, 12, 10098; doi:10.1364/OE.17.010098 (2009).
7. H.W. Choi et al., J. Vac. Sci. and Technol. B: Microelec. and Nano. Struct., 23,1, 4–6; doi:10.1116/1.1843826 (2005).
8. X. Tao et al., Opt. Lett., 36, 7, 1062–4 (2011).
9. J.W. Cha et al., J. Biomed. Opt., 15, 4, 046022; doi:10.1117/1.3475954 (2010).
10. J. Palero et al., Opt. Exp., 18, 8, 8491–8 (2010).
11. K. König et al., Laser Phys. Lett., 8, 6, 465–468; doi:10.1002/lapl.201110014 (2011).
John Girkin is director of the Biophysical Sciences Institute, Durham University, Science Laboratories, South Road, Durham, DH1 3LE, UK; e-mail: [email protected]; www.bsi.dur.ac.uk.