AFM is a well-established surface-analysis tool, but researchers are also using it to manipulate matter on the molecular and atomic scale.
Since first coming onto the scene in 1986, atomic-force microscopy (AFM) has matured into an essential tool for imaging, measuring, sensing, and manipulating surfaces in cell biology, semiconductor manufacturing, and materials science. With the current push for more accurate surface analysis at micron and submicron levels, AFM has received a boost from the nanotechnology world, where it is being used to address several nano-based materials challenges in electronics, telecom, biomed, automotive, aerospace, and energy applications. End users are recognizing AFM's inherent abilities to image and manipulate features as small as a carbon atom in thin- and thick-film coatings, ceramics, composites, glass, metals, polymers, semiconductors, and other materials, and product developers have responded with a host of devices that enhance the ability to use AFM for nanoscale applications.
Invented in the early 1980s by Gerd Binning and Heinrich Rohrer of IBM Zurich, who won the Nobel Prize in 1986 for their work, AFM has its roots in scanning tunneling microscopy (STM), also developed by Binning and Rohrer. It is one of several scanning-probe microscopy techniques, which are based on the interaction between a submicroscopic probe and the surface of a material. In addition to being able to image features ranging from 0.25 to 80 nm in diameter, AFM offers several advantages over STM and other microscopy techniques in both laboratory and industrial environments. Unlike STM, AFM does not require the sample to conduct electricity.
It can operate in normal room temperatures, while STM requires special temperatures and other conditions. The magnification power of AFM, which can exceed 1000:1, rivals that of transmission electron microscopes (TEMs) and scanning electron microscopes (SEMs). In addition, AFMs are capable of a wide field of view (similar to SEM) and extreme vertical resolution (like TEM), but can also image samples in air and liquids without the sample preparation necessary with these other techniques.
"AFM really has made possible the revolution we are seeing right now in nanotechnology," said Stefan Zauscher, assistant professor of mechanical engineering and materials science at Duke University (Durham, NC). "It was among the first tools to manipulate matter on the molecular and even atomic scale and to visualize structures on the nanoscale. Conventional techniques such as SEM and TEM require enormous sample prep and you aren't looking at the actual sample but a coated sample in a high-vacuum environment."
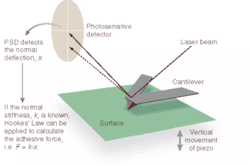
A typical AFM uses a sharp probe or tip attached to a stylus to raster scan a sample surface, imaging the topography of the surface (x, y, and z) and creating a three-dimensional "map" in the process (see Fig. 1). The AFM head uses an optical detection system in which the tip (typically made from Si3N4 or Si) is attached to the underside of a reflective cantilever and extended down from the end of the cantilever. A diode laser is focused onto the back of the reflective cantilever. As the probe scans the sample surface, moving up and down with the contour of the surface, the laser beam is deflected off the attached cantilever into a dual-element photodiode. A photodetector measures the difference in light intensities between the upper and lower photodetectors and converts it to voltage. Feedback from the photodetector enables the tip to maintain constant force on, or constant height above, the sample.
"All the electronics in the feedback scheme have to be designed and made and put together in such a way as to minimize electronic noise," said Michael Serry, senior applications scientist, strategic and technical marketing, at Veeco Instruments (Santa Barbara, CA), the leading supplier of AFMs worldwide. "The same is true of mechanical, optical, and acoustic noise. A well-made AFM is one in which the designers and manufacturer go through an awful lot of fine-tuning to ensure that each and every component is as low-noise as they can find it or make it."
While AFM began as a contact-mode technique, noncontact AFM emerged in the late 1980s in an effort to more accurately image biological samples, which are difficult to scan using contact mode because they are often soft and can be damaged easily. In noncontact mode, the cantilever oscillates close to its resonant frequency at a small distance (1 to 10 nm) above the surface. Long-range attractive forces induce changes in the amplitude, frequency, and phase of the cantilever and maintain a constant distance during scanning. Because the forces on the sample are much lower than in contact mode, even the softest samples can be imaged without damage.
Tapping-mode AFM, introduced in 1993, has become the most popular form of AFM for imaging applications. In this mode, the cantilever is made to vibrate, oscillating at its resonant frequency and gently tapping the sample surface at intermittent (typically 300 kHz) intervals during scanning.1 Like contact-mode AFM, tapping mode allows three-dimensional visualization, individual feature measurements, and statistical measurements such as surface roughness. Tapping mode and contact mode also enable derivative modes of surface characterization, giving rise to images beyond topography that reveal electrical, magnetic, mechanical, chemical, and other unique sample properties.
"Tapping mode decreases the lateral stresses on a surface so you can image much more delicate materials, such as polymers and biological samples," Zauscher said. "And that is just the imaging application."
Outside the laboratory
In fact, although AFM was originally used primarily for imaging, it has evolved into a metrology tool as well, particularly for quality assurance applications in semiconductor and data-storage manufacturing (see Fig. 2). According to Serry, nearly every major semiconductor and magnetic-data-storage company in the world relies on AFM for imaging and failure analysis.
In the last few years, Veeco has broadened its investment in AFM to meet growing interest in nanomanipulation, nanolithography, and molecular-level force spectroscopy and has introduced several new AFM products aimed at these applications. In fact, for many researchers and technology developers, the most exciting trend in nanotechnology is the ability of AFM to truly manipulate matter on molecular and atomic scales. Techniques such as dip-pen nanolithography, invented four years ago by Chad Mirkin and colleagues at Northwestern University (Evanston, IL) and being commercialized by NanoInk (Chicago, IL), are leveraging the inherent lithographic capabilities of AFM and opening new doors for this technology in materials science, such as nanoscale fabrication. Dip-pen nanolithography is a direct-write patterning technique in which a molecule-coated probe tip deposits material onto a surface much like a fountain pen.
"We are getting to the point where you can manipulate surfaces on the nanoscale by drawing on them and putting molecules down where we want them," said Zauscher, who is collaborating with colleagues in the Adaptive Systems and Structures laboratory at Duke to build more precise AFMs for nanolithography. "We make templates onto which we can address other molecules from solution and use catalytic coatings on the AFM tip to create very local chemical reactions. You can first image and then place a feature on the surface this way, which allows us to explore chemistry and chemical physics on the molecular-length scale."
Atomic force microscopy has also been used extensively in biomedical research, but here the technology has had a harder time making the transition to applications outside the research laboratory.
Unlike other scanning-probe microscopes, AFMs are not dependent on the conductivity of the sample being scanned, which means the process can be carried out in ambient air or liquid environments, a critical feature for biological applications. According to Zauscher, in the last 10 years AFM has made major inroads in biology and life sciences because it allows direct observation of cell surfaces and proteins on surfaces or on water, in vivo. For example, scientists at Virginia Tech (Blacksburg, VA) are using a modified form of AFM to measure sticking efficiencies at the nanoscale and have developed a specialized cantilever that allows them to study microparticle attractions.2 In experiments, they have observed at subatomic levels the efficiency of the attachment of bacteria to silica surfaces. Their goal is to develop a new method for predicting how bacteria and other contaminants can be transported in groundwater.
Outside of the research laboratory, however, AFM has yet to find a "killer app" that will expand its use in the biomedical field. But there are indications that the technique is beginning to be used more in applied research through collaborations in fields such as pharmacology. Scientists at Carnegie Mellon University (Pittsburgh, PA), for instance, are using AFM to investigate molecular scale processes underlying the formation of insoluble, protein-based plaques associated with Alzheimer's disease.3 Results of this work suggest that AFM could lead to a better understanding of the disease process and help guide development of new diagnostic and treatment approaches. The research was done in collaboration with scientists at Eli Lilly and the Washington University School of Medicine and supported by the National Institutes of Health.
"Because AFM provides three-dimensional topographical information at the nanoscale, it could prove important in assessing the potential usefulness of molecules like antibodies to effectively inhibit protein aggregation associated with Alzheimer's," said Tomasz Kowalewski, assistant professor of chemistry at Carnegie Mellon. "And because AFM probes the physical state of proteins, it could really assist in understanding conformational diseases, which traditionally have been difficult to fight."
On the commercial front, AFM is part of a new product that can detect viruses earlier and more quickly than other methods and perhaps pave the way for truly clinical applications of AFM (see Fig. 3).4 Developed by BioForce Nanosciences (Ames, IA) and researchers at Des Moines University (Iowa), the "ViriChip" is a silicon chip printed with droplets of antibodies that act as "landing pads" for viruses, which selectively attach themselves to certain antibodies. Once the viruses have landed on a particular droplet, they can be detected using an AFM. In experiments, this technique detected six different strains of a virus called coxsackievirus B, which causes symptoms ranging from mild cold to death and is one of the key factors in failed heart transplants.
"In principle you can fit thousands of different antibodies on one chip and use it to test for thousands of different viral infections simultaneously, using just one sample from a patient," said Eric Henderson, founder and chief scientist at BioForce. "It also means the results will come back in record time and further studies can be carried out on the unperturbed sample using more conventional, if slower, methods. The technique is currently being used by researchers, and we hope it will be available for doctors and hospital pathology labs in the next two years."
REFERENCES
- M.Serry, Laser Focus World (June 2002).
- www.brightsurf.com (Sept. 10, 2003).
- Kowalewski et al., J. Molecular Biology 335(4) 997 (Jan. 23, 2004).
- S.R. Nettikadan et al., Nanotechnology 15, 383 (March 2004).