Chemical imaging reveals more than the microscope
Patrick J. Treado
Combining high-performance digital imaging with spectroscopic analysis creates new chemical imaging tools.
Chemical imaging combines conventional imaging with spectroscopy. In conventional imaging, a macroscopic or microscopic scene is recorded and stored as a series of intensity values at each image element or pixel. Many research and industrial applications use electronic imaging to determine the morphology, geometry, or architecture of a sample or object, often following dynamic events in real time. In traditional analytical spectroscopic techniques such as Raman and/or infrared spectroscopies, the chemical composition is determined by dispersing the light absorbed or emitted by a sample. In these measurements, the data usually take the form of a series of intensity values at various wavelengths in a range.
Both areas--imaging and spectroscopy--continue to be impacted by technological innovations that have made possible faster acquisition of superior-quality data. For example, high-performance, silicon charge-coupled-device (CCD) cameras have brought the benefit of low-noise, multichannel data acquisition to both imaging and spectroscopy. Infrared focal-plane arrays now offer the same benefits for applications in the near- and mid-IR spectral region. In addition, interferometers and tunable filters often can replace diffraction gratings in a number of spectroscopic instruments while delivering improved performance.
Because of these technological advances, it is now possible to combine imaging and spectroscopy in a new field called chemical imaging. Over the past several years, this topic has been the subject of research that both developed appropriate instrumentation and demonstrated viable applications. As the advent of commercially available turnkey instrumentation approaches, it is interesting to examine the process of hardware development. In this area, technology transfer and collaboration between industry, academia, and government laboratories has been fruitful (see "Technology transfer creates new opportunities," p. 76).
In chemical imaging, light intensity is recorded as a function of both wavelength and location (see Fig. 1). In the image domain, the data set includes a full image at each individual wavelength. In the spectroscopy domain, a fully resolved spectrum at each individual pixel can be recorded. As a result, the data contains both structural and compositional information, allowing samples to be probed, even dynamically, with unprecedented analytical power.
For example, the distribution of a particular chemical can be precisely imaged throughout a sample or the chemical composition of any structure or point in the sample can be determined. Not surprisingly, the applications for chemical imaging are diverse and are growing rapidly: from visualizing quantitative changes in calcium-ion distributions in living cells in real time to process-imaging applications in industry. Process imaging refers to the use of chemical imaging to monitor the progress or quality of an industrial process in real time--like a high level of machine vision that provides molecular, compositional information in a digital image format.
It is the combination of several technologies, including high-speed detector arrays, novel methods to spectrally filter images, and powerful data-manipulation software that makes chemical imaging practical. The key to chemical imaging is high-speed, parallel data acquisition and manipulation. For example, to image an object with high fidelity at a spatial resolution of 1000 ¥ 1000 pixels and record intensity data at only 100 different wavelengths, 108 individual data points must be recorded.
The newest generation of high-performance CCD cameras have readout electronics that allow data generation at up to several million pixels per second. But these silicon CCDs are limited to use in the visible and near-IR (1 µm) spectral regions. Fortunately, mid-infrared focal-plane arrays developed for military applications are now available commercially at a cost that is no longer prohibitive for many applications. Constructed of materials such as indium antimonide (InSb) or mercury cadmium telluride (HgCd Te), these actively cooled devices allow chemical imaging to be performed even out to the fingerprint region of the infrared spectrum (8-12 µm).
In chemical imaging, successive image frames are acquired at different wavelengths; in low-light applications, several frames can be recorded at each wavelength and averaged to improve the signal-to-noise ratio. The nature of the wavelength tuning depends on the type of spectroscopy being performed. Basically, the light can be spectrally filtered before the sample or after the sample. For purposes of this discussion, these conditions can be thought of as "source tuning" or "image tuning."
Raman and fluorescence imaging typically use a fixed-frequency laser or filtered lamp for illumination in conjunction with image tuning. This, in turn, requires a wavelength-tuning mechanism that will transmit the full image in the aperture to the data-collection device without distortion. Acousto-optic tunable filters (AOTFs) and liquid-crystal tunable filters (LCTFs) have enabled these techniques. Their speed, programmability, and flexibility are often critical. For mid-IR absorption spectroscopy, step-scan Fourier-transform interferometers are now starting to be used for source-tuning applications.
Imaging optics usually consist of traditional optical microscopes, commercial telescopes, and zoom lenses. These are all easily interfaced to the CCD cameras and tunable filters described above. Because of the huge amounts of data involved, chemical-imaging applications often require powerful software for data reduction, processing, and visualization. In the earlier example with 108 data points, a chemical image recorded at 16-bit resolution, and subsequently stored at 32-bit resolution, creates a single data set of 400 Mbytes.
Of course, there has long been image-acquisition software and related hardware (for example, framegrabber boards) for conventional imaging. For applications involving only two or three wavelengths, these products can be adapted using their RGB functions, when present. But applications involving tens or hundreds of wavelengths cannot be handled in this way and require new software--both for data manipulation and chemical image visualization. For example, ChemImage 1.0 from ChemIcon Inc. (Pittsburgh, PA) is a Windows software package that allows the chemical-imaging user to interactively visualize on a personal computer hundreds of megabytes of data, in both image and spectral format, simultaneously.
Chemical-imaging applications
Chemical imaging has its roots in biomedical applications, using fluorescent labels to map concentrations of calcium ions and other metabolites. Newer applications in biomedicine now rely on Raman scattering or IR absorption, which offer the benefit of studying samples without the risk of invasive perturbation from fluorescent dyes and indicators. One example is biopsy of human breast tissue.
Characterizing tissue. Victor Kalasinsky of the Armed Forces Institute of Pathology (Washington, DC), Neil Lewis and Ira Levin at the National Institutes for Health (NIH, Bethesda, MD), and Michael Schaeberle at the University of Pittsburgh have recently collaborated with me on a study to show the utility of chemical imaging to characterize biopsied human breast tissue. This work is targeted at the polymer inclusions sometimes found in augmented breast tissue. These inclusions are believed to be Dacron polyester.
In the implantation process, breast implants are surgically attached to the chest muscle wall with Dacron patches. Until now, potential problems associated with migration of this polyester have received much less attention than the well-publicized issues of silicone leakage and migration.
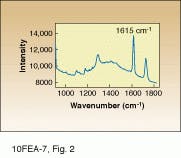
Raman scattering is ideal for this work because Dacron produces a reasonably intense Raman spectrum with cleanly resolved spectral peaks (see
Fig. 2). As a result, molecular-specific images can be obtained without the use of stains or dyes, even in such a complex biomatrix. In this study, the slide-mounted sections were illuminated using the 647.1-nm line of a Coherent krypton-ion laser. Raman-scattered light was collected by a microscope coupled to a 16-bit, red-enhanced, back-thinned CCD with 512 ¥ 512 pixels from Princeton Instruments (Trenton, NJ). Rapid wavelength filtering of the detected light was facilitated by use of an AOTF integrated within the Raman microscope.
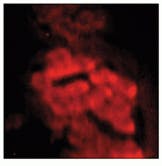
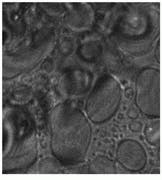
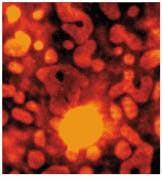
Samples were imaged containing Dacron that had migrated through the breast tissue, forming local aggregates and microstructures (see Fig. 3 and cover). Conventional optical microscopy could not unequivocally determine which of the observed structures were due to Dacron inclusions. One image in Fig. 3 is a bright-field image of the breast-tissue cross section. The other, a Raman image, was taken at 1615 cm-1, which is specific to Dacron. The presence of Dacron was confirmed by using the Raman microscope to take Raman microspectra of these structures at each of the several thousand pixels contained in the chemical image data set.
In the future, this work could be used to determine the three-dimensional structure of these inclusions (globular or fibrous) and to develop quantitative spectroscopic methodologies to analyze natural disease states.
Monitoring coatings. One potential application for chemical imaging using fluorescence spectroscopy involves monitoring thin-film coatings applied to industrial plastics. A specific example in this industry is the monitoring of the paint-priming process of bumpers and other automotive components fabricated from thermoplastic olefin (TPO) polymer.
Thermoplastic olefins are a relatively new class of flexible plastics that are a lightweight, compounded blend of rubber and polypropylene. Rose Ryntz, a technical specialist at the Ford Motor Company Plastic and Trim Products Operations division in Dearborn, MI, explains, "The key to increasing automotive-fuel efficiency is reducing weight. By switching from a conventional urethane to TPO for a bumper, as much as two pounds can be saved." She notes that TPOs already account for 20% of the 4.2 billion pounds of plastics used by the US automotive industry and could reach 50% by the year 2000.
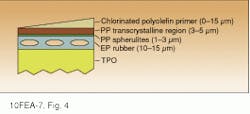
Unfortunately, however, TPOs have very poor surface-adhesion properties and cannot be directly painted. Rather, the surface must first be coated with an adhesion-promoting primer layer consisting of chlorinated polyolefin. The primer is applied by spraying it as a solution in toluene/xylene. This treatment allows the primer to penetrate the rubber-rich subsurface, which is necessary for good adhesion (see Fig. 4). The primed surface can then be painted with conventional enamel paint. The integrity and quality of this primer layer is vital to obtaining a cosmetically acceptable and durable paint finish; the critical parameters are actually primer layer thickness and degree of penetration. Visual inspection is not sufficient to assess this priming process.
Ryntz`s group recently began a collaboration with Hannah Morris and me at the University of Pittsburgh to both investigate the interaction of the primer and TPO and to develop an in-process technique to improve the quality and yield of the priming process. In this work, a small amount of fluorescent dye (DTDC-Iodide) is added to the primer solution. The primed surface is then illuminated with 632-nm light from a HeNe laser, resulting in dye fluorescence observed at 678 nm. The fluorescence is imaged onto a CCD with a LCTF from Cambridge Research and Instrumentation (Cambridge, MA).
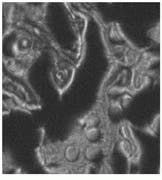
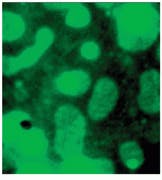
Both microscopic and macroscopic imaging have been used in this study. The microscopic work has led to a better understanding of the primer-TPO interaction, particularly identifying the domain formation of the TPO components and the primer`s tendency to aggregate leading to nonuniform coverage (see Fig. 5).
Macroscopic imaging produced two important results. First, the image intensity at 678 nm is an accurate quantitative indicator of the total amount of primer applied, that is, the thickness of the primer layer. In addition, by imaging multispectrally, the degree of penetration of the primer into the TPO can be established. The minute amount of dye involved (<0.05% by weight) has no perturbative effect on the primer/TPO integrity.
Based on this successful initial study, Ford Motor Co. is funding continued work to more completely understand the microscopic architecture of TPO, using Raman imaging. The next step will be to develop a macroscopic process-imaging system to examine bumpers in-process as part of a pilot program. This will be the next stage in qualifying this approach for full production use.
Successful demonstration of other applications will give researchers new insight into materials problems. The development of chemical-imaging instrumentation brings a novel tool into the analytical arsenal of electro-optical technologies. n
ACKNOWLEDGMENT
Neil Lewis, a consultant to ChemIcon, created the ChemImage 1.0 software.
FIGURE 1. Chemical imaging combines conventional imaging and spectroscopy. Conventional imaging results in information in each pixel based on intensity (top). Spectroscopy produces information at each wavelength (middle). In chemical imaging, however, each pixel contains a diagnostic spectrum, and there is thus a quantitative image at every wavelength of interest (bottom).
FIGURE 2. Raman spectrum of a Dacron
inclusion in biopsied breast tissue cross section shows characteristic peak at 1615 cm-1 that
can be identified in the imaging process.
FIGURE 3. In Raman pathology studies of Dacron in human breast tissue, bright-field image taken through a microscope reveals aggregates (A), while Raman image (taken at 1615 cm-1 ) shows inclusions as bright structures (B).
FIGURE 4. Blow-molding of thermoplastic olefin (TPO) naturally produces strata at the TPO surface (diagrammed in cross section).
The primer must penetrate to the rubber-rich subsurface for good adhesion. Primer coverage and penetration can be monitored with
chemical imaging techniques.
fluorescence spectroscopy involves monitoring thin-film coatings applied to industrial plastics. A specific example in this industry is the monitoring of the paint-priming process of bumpers and other automotive components fabricated from thermoplastic olefin (TPO) polymer.
Thermoplastic olefins are a relatively new class of flexible plastics that are a lightweight, compounded blend of rubber and polypropylene. Rose Ryntz, a technical specialist at the Ford Motor Company Plastic and Trim Products Operations division in Dearborn, MI, explains, "The key to increasing automotive-fuel efficiency is reducing weight. By switching from a conventional urethane to TPO for a bumper, as much as two pounds can be saved." She notes that TPOs already account for 20% of the 4.2 billion pounds of plastics used by the US automotive industry and could reach 50% by the year 2000.
Unfortunately, however, TPOs have very poor surface-adhesion properties and cannot be directly painted. Rather, the surface must first be coated with an adhesion-promoting primer layer consisting of chlorinated polyolefin. The primer is applied by spraying it as a solution in toluene/xylene. This treatment allows the primer to penetrate the rubber-rich subsurface, which is necessary for good adhesion (see Fig. 4). The primed surface can then be painted with conventional enamel paint. The integrity and quality of this primer layer is vital to obtaining a cosmetically acceptable and durable paint finish; the critical parameters are actually primer layer thickness and degree of penetration. Visual inspection is not sufficient to assess this priming process.
Ryntz`s group recently began a collaboration with Hannah Morris and me at the University of Pittsburgh to both investigate the interaction of the primer and TPO and to develop an in-process technique to improve the quality and yield of the priming process. In this work, a
small amount of fluorescent dye (DTDC-Iodide) is added to the primer solu-
tion. The primed surface is then illuminated with 632-nm light from a
HeNe laser, resulting in dye fluores-
FIGURE 5. Microscopic images of DTDC-Iodide tagged thin-film primer on thermoplastic olefin show a comparison of white-light and 678-nm fluorescence images at various film thicknesses [A and B 7.5 µm] and [C and D 15 µm]. Note the aggregation of dye in the rubber-rich domains of the TPO that could not be clearly seen in the white-light reflectance images.
PATRICK J. TREADO is assistant professor, department of chemistry, University of Pittsburgh, PA 15260, and president of ChemIcon Inc., Pittsburgh, PA 15260.
Technology transfer creates new opportunities
As government and university laboratories pursue alternative sources of funding and electro-optics companies increasingly direct their efforts toward diverse commercial markets, technology transfer is becoming very important to the health of our industry and the research community. The brief history of industrial chemical imaging presents an excellent case study of successful technology transfer in action.
Many industries have production limitations and problems that could be solved by chemical imaging. They have the money to pay for cost-effective solutions, but developing a solution from scratch requires an extensive R&D infrastructure with personnel skilled in electro-optics (E-O) technology, physics, and photochemistry, as well as systems integration. Only a few of the very largest companies have the requisite resources to develo¥their own solutions.
On the other hand, academic groups often perform applied research that is only one or two steps removed from such a solution. In other words, one grou¥has the specialized know-how and needs financial resources, while the other grou¥has resources but needs additional, specialized know-how. Technology transfer means bringing these two groups together in a symbiotic way.
One effective way to do this is to create companies with strong ties to both groups. My own company, ChemIcon Inc. (Pittsburgh, PA), was created in 1994 for this purpose. We have strong relationships with both universities and government laboratories and can use these contacts to license new techniques as soon as they become available. At the same time, we have developed a broad network of industrial users and potential users of chemical imaging: companies as diverse as Procter and Gamble, Eli Lilly, Ford Motor Co., and Westinghouse Electric.
These associations can develo¥in several ways. For example, ChemIcon can coordinate a joint collaboration between an academic grou¥and a sponsoring corporation. It also performs contract applied research and supplies complete systems. As a commercial (profit-based) company, it is naturally interested in making technology transfer efficient.
The novel solutions developed by these collaborations still depend on the availability of innovative technology. This leads to the question, "How can electro-optics manufacturers play a more active role in this process?" Many E-O companies are already aware of the importance of this process and support it by loaning and beta-siting expensive equipment at collaborating academic laboratories. For example, our laboratory is collaborating with Chromex (Albuquerque, NM), a Raman-spectrometer manufacturer, to develo¥advanced Raman microprobe technology suitable for in situ microscopy.
In addition, we are currently participating in a preliminary study of the use of Fourier-transform infrared spectrometers for industrial chemical imaging, involving personnel from the National Institutes for Health (NIH), ChemIcon, and Procter and Gamble (Cincinnati, OH). Recognizing the potential importance of the infrared work, Bio-Rad Digilab Instruments (Cambridge, MA) loaned the NIH both a step-scan interferometer and an IR microscope. These are but two examples of a trend that we believe is important and will continue.
P. J. T.