Improvements in electron microscopy and image-processing techniques have enabled Researchers at Lawrence Berkeley National Laboratory (LBNL; Berkeley, CA) to image Brownian motion in molten lead as well as skeletal details of protein cells down to a resolution of 0.8 nm.1, 2
Brownian motion was first observed and documented by Scottish botanist Robert Brown in 1827, using a magnifying glass to examine the motion of wildflower pollen grains in water. The observation was explained in 1905 when Albert Einstein published formulas for the temperature- and size-dependent movement of particles suspended in liquids.
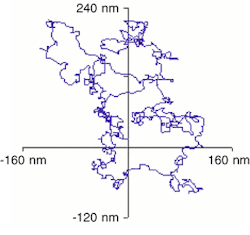
A little over ten years ago, electron microscopy and image-processing technology allowed researchers at the University of Copenhagen (Copenhagen, Denmark) to observe similar movements of nanoscale inclusions of molten lead within the crystalline structure of solid aluminum. A precise analysis to confirm that such motion was largely Brownian was not achieved until last year using the analytical electron microscope at LBNL, which allows imaging while the sample temperature is being varied.The Berkeley researchers accomplished the task by videotaping the movement of the molten lead particles and analyzing the videos on desktop computers using image-analysis software developed in-house. Process steps included grabbing a thousand frames of video, acquiring several thousand data points per frame, following the center of mass of specific particles, and correcting for the drift of the image (see Fig. 1).
The results may help to yield insights into the formation of alloys based on Einstein's theory of Brownian motion, according to Uli Dahmen, who heads the National Center for Electron Microscopy at LBNL. "This may allow us to determine diffusion constants for different alloys by observing the behavior of individual particles," Dahmen said. "By recording particles of known size at, say, five different temperatures, we can calculate the activation energy of the diffusion process."
Nanoskeletons
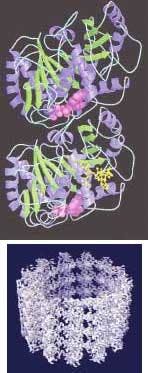
Five years ago, a different research team at LBNL obtained a 2-nm-resolution image of the protein microtubules that help cells to maintain their shape, transport materials, and divide. Last year, they were able to boost that resolution to an 0.8-nm atomic level using a single-particle imaging process of electron microscopy.
The 2-nm resolution method had been based on a helical reconstruction method of image processing, but since the microtubules are not perfect helices, distortions and other defects can plague the visualization process, according to Kenneth Downing, a biophysicist in the LBNL Life Sciences Division.
"To compensate for some of those distortions, we essentially broke each microtubule image into very short segments," he said. "And then we identified the relative orientations of each of these segments with respect to each other."
In addition to aligning very small image segments, the process also entailed averaging large numbers of images to provide an acceptable signal-to-noise ratio for the necessarily low-resolution imaging process. "These are all unstained images, and the exposure that we can use on them is very low because the samples tend to burn up in the beam quite rapidly," he said.
The 0.8-nm-resolution image was produced from 89 microtubule images comprising 1200 segments and about 200,000 tubulin monomers (see Fig. 2). The improved resolution is allowing researchers for the first time to closely study tubulin dimers as they appear naturally in a microtubule, which is expected to improve understanding of basic processes in the life cycle of a cell.
A big help for research into microtubules has been the availability of a 400-kV microscope, which boosts resolution by about a factor of two above what could be obtained with the 100-kV microscopes that were generally available a little over a decade ago. The protein samples are embedded in a thin layer of ice suspended across a hole in the sample platform, and Downing believes that the thicker samples that can be imaged with the higher-voltage microscope provide much better mechanical rigidity.
REFERENCES
- U. Dahmen et al., Proc. Intl. Cong. Elec. Microscopy-15 and Proc. Microscopy and Microanalysis 2002.
- H Li et al., Structure 10, 1317 (October 2002).
About the Author
Hassaun A. Jones-Bey
Senior Editor and Freelance Writer
Hassaun A. Jones-Bey was a senior editor and then freelance writer for Laser Focus World.