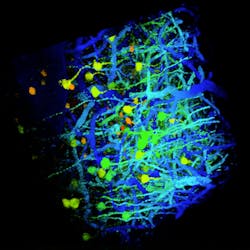
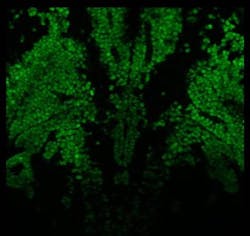
Multiphoton Imaging/Light Sources: New flexibility for deep, nondestructive imaging
ROSY MANSER
Multiphoton microscopy enables deep 3D imaging of biological specimens and plays an established role in life science research, including neuroscience. But approaches and image acquisition technologies that were groundbreaking a decade ago are now reaching their limits. Enhanced understanding of structure and function requires deeper imaging approaches that also minimize tissue damage. Recent innovations in ultrafast lasers provide significant improvements in both depth penetration and efficiency of fluorescence excitation.
You are here
Confocal microscopy, a common technique for life sciences, is routinely used for optical sectioning of cells, tissues, and organisms. Its basis is the scanning of a laser across a sample to cause fluorescence excitation, and subsequent removal of out-of-focus fluorescence—a pinhole located in a conjugated focal plane provides an image only at the plane of focus. Confocal provides a convenient, push-button means of delivering high-resolution 3D datasets of both fixed and live samples.
In 1990, researchers at Cornell University (Ithaca, NY) demonstrated that fluorescence excitation can also be achieved via the simultaneous absorption of two or more photons of longer-wavelength light from a pulsed, femtosecond laser.1 The probability of this event occurring is extremely low except at the plane of focus, alleviating the need for a pinhole to remove out-of-focus fluorescence. With two-photon excitation, the detector captures even scattered emission light from deep inside the sample, and thus the technique can acquire images at significantly increased depth.
In more recent developments, the use of three-photon microscopy has shown significant promise in providing a means of imaging even deeper into biological specimens. Three-photon excitation is achieved by the simultaneous absorption of three photons of light by the fluorophores. The common wavelengths used for three-photon excitation in microscopy are between ~1300 and ~1700 nm. Particularly for neuroscience, this allows access to different regions and structures in the brain without invasive surgery.
For example, in mouse samples, it has been shown that the hippocampus can be imaged through nondestructive, three-photon imaging without the requirement of a GRIN lens,2 and more recently that brain regions can be imaged even through the intact skull.3 Similarly, for the Drosophila neuroscience community, three-photon imaging provides the means to image brain structure in vivo,4 and as such it becomes feasible to perform functional imaging at the single cell level in the whole brain.
Three-parameter balancing
Three-photon microscopy is enabling new and exciting studies, and the range of research benefitting from it has increased along with the commercial availability of suitable microscope systems and ultrafast lasers. To maximize depth and efficiency of multiphoton imaging approaches, however, it is necessary to co-optimize the acquisition pathway. From a hardware perspective, this means the microscope, scan head, detection path, and femtosecond laser must be refined and designed to work together.
Optimizing lasers for multiphoton microscopy is a challenging task that requires consideration of several interdependent parameters that can impact both sample and image quality. The key is to design a laser that optimizes excitation by providing the best balance among peak power, average power, and repetition rate.
Peak power—the pulse energy divided by the pulse duration—is the most important parameter in defining excitation efficiency and therefore level of fluorescence signal. For two-photon excitation, fluorescence signal is the product of peak power and average power, and for three-photon excitation, it is the product of peak power squared and average power. Thus, for the most efficient means of fluorescence excitation and therefore the deepest imaging approaches, high peak power is extremely important.
Because higher average power means more fluorescence signal, it might be tempting to assume that the greater the average power, the better. But in life sciences, overheating a sample can change the physiology and damage the sample—and average power produces this heating effect (see Fig. 1). The damage threshold varies significantly among sample types, species, and preparation. As an example, though, the average power threshold for imaging mouse brain through a craniotomy is ~250 mW.5 Imaging at higher average powers risks damaging the specimen.The third important parameter of the femtosecond laser for multiphoton imaging is repetition rate, defined as the number of emitted pulses per second. When teamed with a scanning microscope, it is the laser’s repetition rate that determines the physical maximum frame rate of the overall system. Because multiphoton imaging is often done using live samples, a repetition rate sufficiently high to enable usable acquisition speeds is important.
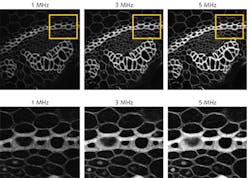
Assuming an image is acquired at 512 × 512 pixels, with one laser shot per pixel and a 20% over-scan, a repetition rate of 1 MHz enables a frame rate of >3 frames per second (fps). For higher frame rates—useful for following fast events or decreasing overall imaging time—higher repetition rates are required (for example, 5 MHz provides >15 fps). It should be remembered, however, that an increase in repetition rate causes an increase in average power, which can damage a sample. As such, the higher the laser’s repetition rate, the lower the possible peak power. Having the flexibility to change the repetition rate to suit each experiment is the optimal solution, giving the user the ability to select the best combination of parameters for each image acquisition (see Fig. 2).6Laser sources
A wide range of light sources has been used for multiphoton imaging. Historically, the titanium sapphire (Ti:sapphire) laser was the key laser technology adopted for two-photon imaging. The high reliability and ease of use of this platform set it aside from other solutions, and when paired with either a home-built or a commercially available two-photon microscope, it provides turnkey ease to reach multiphoton capability in any lab. Additional developments further improve the performance of Ti:sapphire lasers—for instance, automated pre-compensation of pulse duration enables improved image quality and depth.
As the availability and utilization of different dyes and genetic fluorescence tags continue to increase, it is useful to increase the excitation range of the femtosecond laser. To reach longer wavelengths, Ti:sapphire lasers can be combined with an optical parametric oscillator (OPO), which increases the tuning range to the mid-infrared (mid-IR), enabling more optimal excitation of red dyes.
More recently, the OPO has been teamed with a number of different laser types, including bulk-based and fiber lasers, to further increase the tuning range. The benefit of combining the bulk- or fiber-based laser with the OPO is that the seed line (~1040 nm) is still available. This enables optimal excitation of multiple fluorophores by using both wavelengths at once, which can significantly improve image quality during multicolor acquisitions.
The significant downside of both the Ti:sapphire and the bulk- or fiber-laser-driven OPO is the repetition rate that is fixed at ~80 MHz. Since repetition rate and average power are inextricably linked, this limits the potential peak power because of the average power threshold. Thus, lasers with high, fixed repetition rates limit the excitation efficiency of fluorophores, which in turn limits the penetration of light into the sample.
At the other end of the repetition rate spectrum are the very low-repetition-rate Ti:sapphire amplifiers or high-energy optical parametric amplifiers (OPAs). These lasers provide tremendous peak power, which is ideal for optimal excitation and great depth penetration, but the repetition rate is too low (<1 MHz) to enable frame rates that are suitable for the majority of life science imaging requirements.
For three-photon imaging, the laser parameter relationships are the same—although the range of commercially available lasers is much smaller. Some researchers have used high-repetition-rate, low-energy, oscillator-driven OPOs, but because the low peak power limits excitation efficiency, penetration depth is also limited. The high-energy OPA, driven by lasers manufactured for machining, provides good excitation efficiency because of its high peak power, but limited frame rate because of its low repetition rates (~1 MHz). A third class of laser, the low-energy OPA, offers wide tuning ranges and provides a range of repetition rates (and hence frame rates), but the excitation efficiency and thus depth is limited by the relatively low peak power.
New options meet researchers’ wish lists
Recent developments in fiber laser technology are now providing unique parameter combinations that ensure optimal fluorescence excitation as well as frame rates suitable for life science imaging (see image at top of this page). These technologies, such as the KMLabs Y-Fi NOPA and OPA (see Fig. 3), provide tunable repetition rates from 1 to >5 MHz as well as high peak powers, wide tunability, and availability of two wavelengths simultaneously, which make them ideally suited for multiphoton applications. Researchers at Cornell University stated in 2018 that “wavelength-tunable sources at >900 nm (the wavelength window of interest for a large number of brain imaging applications) that can provide more than 50 nJ pulse energy with repetition rate tunable within the range of 1 to 10 MHz do not exist currently, but are needed for optimum deep-tissue two-photon imaging.”6 The recent innovation of fiber laser solutions meeting these criteria means that this statement is no longer true. The researchers now have what they need to optimize their efforts to image deep into tissue.As life scientists continue to push the boundaries of what can be achieved using multiphoton microscopy, developments in imaging technologies and laser sources play key roles in facilitating their work. These recent innovations in ultrafast fiber lasers provide the long-desired flexibility to optimize the balance of parameters for every experiment. The choice of how to use the additional fluorescence signal provided by these laser sources (to image deeper, more gently, or more quickly) will depend on the scientific question, but it’s a choice that has simply not been available before.
REFERENCES
1. W. Denk, J. H. Strickler, and W. W. Webb, Science, 248, 4951, 73–76 (1990).
2. N. G. Horton et al., Nat. Photonics, 7, 205–209 (2013).
3. T. Wang et al., Nat. Methods, 15, 789–792 (2018).
4. K.-J. Hsu, Y.-Y. Lin, A.-S. Chiang, and S.-W. Chu, bioRxiv (2018); http://dx.doi.org/10.1101/339531.
5. K. Podgorski and G. Ranganathan, J. Neurophysiol., 116, 3, 1012–1023 (2016).
6. K. Charan, B. Li, M. Wang, C. P. Lin, and C. Xu, Biomed. Opt. Express, 9, 5, 2304–2311 (2018).
Rosy Manser, Ph.D., is European Sales Manager in the Life Sciences Division at KM Laboratories (KMLabs), Boulder, CO; e-mail: [email protected]; www.kmlabs.com.