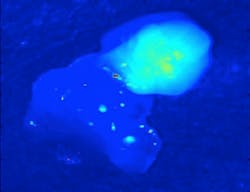
Translational Research/Near-infrared Fluorescence: Intraoperative systems leverage parathyroid autofluorescence
The U.S. Food and Drug Administration (FDA) has cleared for marketing two devices based on a principle discovered by Vanderbilt University (Nashville, TN) researchers. The approvals are expected to transform how thyroid and parathyroid surgeries are performed in the United States, even as the researchers work to improve the technology.
Scientists in the lab of Anita Mahadevan-Jansen, Professor of Biomedical Engineering at Vanderbilt, first reported that the body’s four parathyroid glands, each as small as a grain of rice, autofluoresce under near-infrared (near-IR) light in 2008. “When we discovered this strong autofluorescent signal in the parathyroid, we thought it was stray light,” says Mahdevan-Jensen. “But the consistency of the signal proved that it was real.”
Surgery is the standard treatment for parathyroid disorders, and surgeons normally find it hard to visualize these structures and distinguish them from nearby tissues. But visual examination and surgical experience have been the only assessment means available. Incomplete resection of diseased parathyroid tissues is common today, and in about 20% of thyroid surgeries, healthy parathyroid tissues are accidentally removed, resulting in complications for patients and unnecessary costs. Near-infrared autofluorescence (NIRAF) gives surgeons powerful new noninvasive, label-free assistance intraoperatively.
A decade of development
Mahadevan-Jansen and her team developed the technology at the Vanderbilt Biophotonics Center. Their original setup used a 785 nm diode laser to deliver 80 mW to tissue with a 400 μm spot size, a fiber-optic spectrometer with a spectral resolution of 10.5 nm full-width at half-maximum (FWHM) to detect fluorescence spectra, and filters to keep the excitation light from the detector. With this, they were able to determine that the intensity of the parathyroid’s fluorescence is significantly greater than that of all other tissues in the neck and proved that the technique is able to consistently detect parathyroid tissue with near 100% accuracy (see figure).1
In 2018, the team developed and investigated a clinical prototype called PTeye that achieved 96.1% accuracy in in vivo tests with human patients undergoing thyroidectomy and/or parathyroidectomy at Vanderbilt University Medical Center and Ohio State University Medical Center.2
AiBiomed (Santa Barbara, CA), a spinoff of nano-IR company Anasys Instruments (which was acquired in 2018 by Bruker), holds the license from Vanderbilt to produce and market PTeye. The company is commercializing the device, which incorporates a 785 nm laser source (delivered at 20 mW), a detector, a detachable fiber-optic probe that can be sterilized, a foot pedal to activate NIRAF measurements, and a user interface designed to be friendly. Critically, internal circuitry allows the system to detect NIRAF without interference from ambient lights, facilitating integration into existing workflows. The unit cues surgeons in real time using audio as well as a visual display. AiBiomed will exhibit at the 2019 American Association of Endocrine Surgeons meeting—the same event at which the Vanderbilt team first reported its work in 2008.
Separate and concurrent
The other device to be FDA-cleared, Fluoptics’ (Grenoble, France) Fluobeam 800 Clinic Imaging Device, was previously cleared to serve as an adjunctive method for visual assessment of tissue perfusion. For this device, the FDA reviewed data from five peer-reviewed studies, including one that compared rates of postoperative hypocalcemia (PH)—that is, a reduction in blood calcium that occurs when healthy parathyroid tissue is inadvertently removed. Only 5% of patients who had the surgery assisted by Fluobeam 800 experienced fluctuating PH afterward compared with 21% of patients whose surgeries did not use NIRAF.
FDA reviewed Fluobeam 800 and PTeye concurrently but separately under its de novo premarket regulatory pathway available for novel devices that have no prior legally marketed counterparts and are considered to pose little to moderate risk. The devices are intended to assist, but not replace, experienced visual assessment and biopsy and only to assist in locating parathyroid tissue or glands.
New frontiers
Wanting to further improve parathyroid surgical guidance, the Vanderbilt researchers recently developed the Overlay Tissue Imaging System (OTIS).3 Their goal was to obviate the need for a remote display and mitigate potential for incorrect interpretation of the PTeye’s visual cues. They designed OTIS to project NIRAF signal as visible imagery directly onto the operative field. OTIS includes a 785 nm diode laser, an image-collection unit (including a filtered near-IR camera), a visible-light projector, optics for coupling and focusing, and a laptop for image processing. At a distance of 50 cm from a 5 × 6.25 cm operative field, OTIS exhibited 176 μm spatial resolution.
The researchers determined that OTIS will need continued work—for instance, because ambient operating-room lighting proved problematic for the system. Further work is also necessary to effectively reveal parathyroid glands located beneath layers of tissue, they say.
Meanwhile, though, Binita Ashar, MD, director of the surgical devices division in the FDA’s Center for Devices and Radiological Health, expects the newly approved devices will provide “valuable information to help preserve healthy tissue or to remove diseased tissue.”
REFERENCES
1. C. Paras et al., J. Biomed. Opt., 16, 6, 067012 (2011).
2. G. Thomas et al., Thyroid, 28, 11, 1517–1531 (2018).
3. G. Thomas et al., Surgery, 165, 114–123 (2019).
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.