New slant on superresolution microscopy at Sandia unlocks secrets of plague
Albuquerque, NM--Researchers at Sandia National Laboratories have developed a superresolution-microscopy technique that is answering long-held questions about exactly how and why a cell's defenses fail against some invaders, such as plague, while successfully fending off others like E. coli. The approach is revealing never-before-seen detail of the cell membrane, which could open doors to new diagnostic, prevention, and treatment techniques.
The technique builds on superresolution capabilities developed in recent years, but goes another step by adding dual-color capabilities to the relatively new stochastic optical reconstruction microscopy, or STORM.
The membrane of a cell not only provides structure and houses the cell's interior, it also regulates movement of materials in and out of the cell, controls adhesion to other objects, and coordinates the cell's communications and subsequent actions through signaling. Receptor proteins on the surface of immune cells, known as toll-like receptors (TLRs), are tasked with recognizing intruder antigens. The TLR4 member of this receptor family responds to certain types of bacteria by detecting lipopolysaccharides (LPS) present on their surface. TLR4 proteins then alert the cell and activate an immune response.
Simultaneous imaging of two receptor types
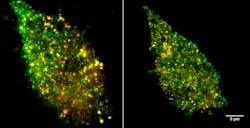
Using the new imaging technique, Sandia researchers Jesse Aaron, Jeri Timlin, and Bryan Carson discovered that TLR4 proteins cluster in the membrane when confronted with LPS derived from E. coli, which increases cell signaling and response. Interestingly, LPS derived from the bacteria that cause plague, Yersinia pestis, do not cause the same effects. This could explain why some pathogens are able to thwart the human immune system.
The plague studies marked the first time such small events have been imaged and compared, the Sandia researchers said. The technique enables the Sandia team to simultaneously image LPS and TLR4 receptors on the membrane.
In 2009, the National Institutes of Health awarded Timlin a five-year, $300,000-a-year innovation grant. Next on the team's agenda is developing the capability to image live cells in real time using spectral Stimulated Emission Depletion, or STED. "We're working toward using a version of superresolution that's much more live-cell friendly, and extending that in terms of what colors are available to do multiple colors, while maintaining the live-cell friendliness. I see this as a beginning of a long development in this type of imaging technology," Timlin said.
Potential applications likely will expand as the technology reveals previously unattainable details of cell signaling. Eventually, the Sandia team would like to be able to visualize protein/protein interactions. The technology has exciting potential in immunology and drug discovery. Improved imaging could show the mechanisms viruses use to invade cells, which might lead to drugs that would block entry.

John Wallace | Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.