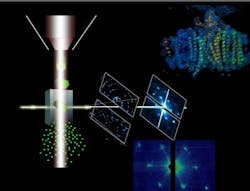
X-ray free-electron laser discerns structure of protein crystals
| A protein beam-injector (left) ejects photosystem I nanocrystals (vertical stream); the stream of crystals interacts with the LCLS free-electron laser X-ray beam. |
Menlo Park, CA--Scientists using the x-ray free-electron laser (FEL) at the Department of Energy's SLAC National Accelerator Laboratory have made progress toward unraveling the molecular basis of life. Known as the Linac Coherent Light Source, the x-ray FEL allowed the scientists to determine structures of biomolecules based on diffraction from protein nanocrystals too small to see under an optical microscope.1, 2
Video-rate operation
In the technique, a small aerojet nozzle provides a fully hydrated constant stream of nanocrystals, both supplied from an interdisciplinary research team from Arizona State University (ASU; Tempe, AZ). The international team of nearly 90 researchers collected more than 3 million diffraction-pattern snapshots (at a rate of 30 diffraction images per second) just before the crystals disintegrated in the femtosecond X-ray pulses;
"From the beginning, the resolution of images recorded by biologists has been limited by damage due to the radiation used," said physicist John Spence, a professor at ASU and one of the lead authors of the studies. "But what happens if a pulse of imaging radiation is used that terminates before damage begins, yet contains sufficient photons to generate a useful scattering pattern?"
Thirty percent of all proteins are not soluble but are embedded in membranes. "These membrane proteins, major players in the function of all living cells, are the targets for more than 50% of all drugs, yet, scientists have a lack of information on their structure and function," said Petra Fromme, also of ASU, and the second author of the nanocrystallography work.. "While more than 60,000 structures of soluble proteins have been determined to date only 256 membrane proteins structures are known and only four of them are human membrane proteins."
For the nanocrystallography experiments, which took place in December 2009 at SLAC, Fromme's lab at ASU supplied nanocrystals of photosystem I, a large membrane protein complex that acts as a biosolar energy converter in oxygenic photosynthesis.
REFERENCES:
1. Henry N. Chapman et al., Nature, Vol. 470, p. 73, 03 February 2011; doi:10.1038/nature09750.
2. M. Martin Seibert et al., Nature, Vol. 470, p. 78, 03 February 2011; doi:10.1038/nature09748.
Subscribe now to Laser Focus World magazine; it's free!
About the Author
John Wallace
Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.