Noninvasive optical window created in the skull allows brain imaging
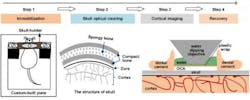
A noninvasive approach for creating an optical window in the skull to enable the brains of living mice to be imaged has been demonstrated at the Huazhong University of Science and Technology (Wuhan, China).1 Dan Zhu and her coworkers tested the use of optical clearing agents (OCAs) that they applied to the bare skulls (hair and skin removed) of living mice. After treatment with OCAs, the skull becomes transparent within minutes, thus forming a visible window of the cortex.
Combined with two-photon microscopy, this technique allows imaging of the fine structures of neurons, glia, and the microvasculature in the mouse brain. Given its easy handling, safety, repeatability and excellent performance, this method has promise in neuroscience research.
Chronic observation and manipulation of cells in the cortex is critical to studies of brain structure and function. However, the strong scattering caused by the skull over the cortex limits the penetration depth of light in tissues and thus hinders the observation of fluorescently labeled neuronal structures and microvasculature. To overcome this obstacle, various cranial window methods have been developed, including the open-skull glass window, the thinned-skull cranial window, and their variants. But these methods present limitations.
RELATED: Bone-clearing method allows observation of the stem cells inside
The optical clearing technique reduces the scattering of tissues and has become an important tool for optical imaging in biomedical research. However, the current optical clearing method is widely used only in ex-vivo (in other words, not in the living body) studies of various tissues and organs; there are few studies on how to make the living tissues transparent.
Increasing skull transparency
Zhu first proposed the study of an in-vivo optical clearing technique. In the early stages, she was focused on the research of different types of skin tissue. Tonghui Xu, Zhu's colleague, has been engaged in the research of cortical neuroimaging in mice; for in-vivo cortical imaging, the turbid (light-scattering) skull becomes a great bottleneck.
After communicating with Xu, Zhu saw the need for neuroscientists to have a window into in-vivo brain tissue and began to engage in the research on optical clearing of skull tissue. After six years of hard work, they developed an effective, safe, and switchable skull optical-clearing window.
The resulting tissue optical-clearing technique relies on the use of the clearing agents collagenase and glycerol for young mice, and EDTA disodium and glycerol for older mice with higher bone mass. In both cases, the glycerol is used as an index-mating fluid.
Through the resulting window, the image contrast and imaging depth are significantly improved, allowing imaging of the cortical structures at synaptic resolution. This technique holds great promise for the studies of brain structure and function in physiological or disease states.
Source: https://eurekalert.org/pub_releases/2018-02/cioo-soc022818.php
REFERENCE:
1. Yan-Jie Zhao et al.,Light: Science & Applications (2018) doi: 10.1038/lsa.2017.153.
About the Author
John Wallace
Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.
