Plug-and-play fiber-optic sensors based on engineered cells enable in vivo monitoring of neurochemicals
When a neurologist proposed monitoring the dynamical concentrations of neuromodulators in epilepsy in vivo (within live organisms or tissues) to help inspire drug discovery for the neurological condition, Lingjie Kong, an associate professor at Tsinghua University, and colleagues did a quick search to see whether any available sensors could meet the requirement of high chemical specificity.
None did, even sensors made of advanced materials, so they turned to nature for a little inspiration.
“Novel genetically encoded fluorescent indicators (GECIs), such as G protein-coupled receptor activation-based (GRAB) sensors and cell-based neurotransmitter fluorescent engineered receptors (CNiFERs), are of high specificity because they take advantage of the evolution of neurological receptors,” says Kong.
But GECIs are generally expressed through viral infections, which take approximately two weeks. “Even worse, it’s much longer if you plan to cultivate transgenic animals, considering the reproductive cycle of model animals,” he adds. “Moreover, for different animal species, labor-intensive work is needed to explore strategies for efficient viral infection. On the other hand, it’s difficult, if possible, to monitor neurochemical dynamics within the blood circulation system in vivo with current GECIs.”
Plug-and-play fiber-optic sensors
Instead, Kong and colleagues opted to assemble the key components of fluorescence sensors in engineered cells and culture these cells on fiber tips.
“The chemical specificity is unprecedented—as high as that inside real organisms, and plug-and-play sensing is promising independent of animal models,” says Kong. “As far as potential applications in humans, after an ethics approval, it’d be much easier and more secure than fluorescence sensing by gene manipulation.”
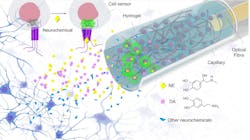
They developed their plug-and-play fiber-optic sensors based on engineered cells (FOPECs) for monitoring neurochemicals in vivo by integrating genetically engineered cells that express GRAB neurotransmitter sensors with optical fibers for real-time sensing (see Fig. 1 and video). This eliminates the time-consuming procedures of viral infection and also ensures high specificity. “To do it, we embed these engineered cells within biocompatible hydrogels and load the mixture around the tips of optical fibers for sensing,” he says.
The trickiest part involves assembling the engineered cells on the fiber probes and delivering them safely to the target regions. Cells are notoriously fragile, so they need to be protected during the probe implantation to maintain their superior sensing capability. This is why Kong and colleagues embed the engineered cells inside biocompatible hydrogels and load the mixtures around the fiber tips with the protection of capillary windows.
Real-time sensing of neurochemicals at high specificity
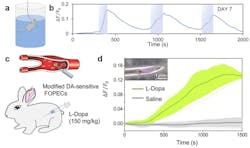
In what’s believed to be a world’s first, Kong and colleagues’ FOPECs enable real-time sensing of neurochemicals at high specificity for various species of animals and organs—immediately after acute implantation (see Fig. 2).
“We’ve demonstrated FOPECs in plug-and-play, real-time monitoring of norepinephrine, dopamine, and adenosine triphosphate in mice, rats, rabbits, and chicken, under various physiology and pathology conditions,” Kong says. “The dynamics of neurochemicals are monitored in both the brains and blood circulation systems of freely moving animals. We expect FOPECs to not only benefit neuroscience, but also speed drug discovery.”
Future improvements
To improve the performance of FOPECs, “future efforts in fluorescent protein engineering could improve the sensor’s brightness and affinity,” says Kong. “Beyond this, more work can be done to make FOPECs less invasive, more biocompatible, and easily replaceable.”
For other applications, the superior performance of FOPECs makes it possible to explore many open biological questions. “The brain-wide spatio-temporal dynamics of neurochemicals is an essential piece of understanding neural mechanisms in health and diseases,” says Kong. “Because our FOPECs provide the high-specificity solution for neurochemical sensing in vivo, the next step is to study the neurochemical dynamics across the entire brain at high spatio-temporal resolutions.”
Beyond this, multifunction FOPECs are desired for simultaneous monitoring of multiple neurochemicals, which can be accomplished via wavelength multiplexing of engineered cells with multiple GRABs in various colors.
FURTHER READING
B. Zhou et al., Sci. Adv., 9, 22 (Jun. 2, 2023); https://doi.org/10.1126/sciadv.adg0218.

Sally Cole Johnson | Editor in Chief
Sally Cole Johnson, Laser Focus World’s editor in chief, is a science and technology journalist who specializes in physics and semiconductors. She wrote for the American Institute of Physics for more than 15 years, complexity for the Santa Fe Institute, and theoretical physics and neuroscience for the Kavli Foundation.