Quick two-photon optogenetics fix enables all-optical physiology
To map the functional connectivity of neural circuits in vivo (inside a living animal), two-photon optogenetics is indispensable because it allows neuroscientists to noninvasively manipulate neural activities at high spatial resolution. It does come with a few challenges, but researchers at Tsinghua University in China discovered a quick fix to enable all-optical physiology.
“Considering that functional imaging of neural network activity only provides correlations of neural connectivity, the combination of functional imaging and optogenetics, called ‘all-optical physiology,’ is attracting great interest for its potential to reveal the causality of functional connectivity,” says Lingjie Kong, an associate professor in the Department of Precision Instruments, who recently presented his group’s work at the 2022 Biophotonics Congress.
Kong’s group is devoted to developing novel tools to enable neuroscience studies, and in 2018 he was honored by MIT Technology Review as one of the “35 Innovators Under 35” in China for his work with neuroimaging that enables real-time observation of neural activity across different brain regions within conscious animals.
“We’ve developed a high-speed volumetric imaging system based on an ultrasonic lens, a deep tissue imaging system based on multi-pupil adaptive optics, and a centimeter-scale micrometer-resolution macroscope of gigapixel throughput,” Kong says. “And we realized the urgent need for all-optical physiology, which currently lacks efficient and precise manipulation techniques.”
Two-photon optogenetics and computer-generated holography
To ensure selective simulations of neurons, “two-photon optogenetics is desirable, because effective simulations only occur around the laser focus, where the photon intensity is high enough,” explains Kong. “In practice, two-photon optogenetics based on computer-generated holography (CGH) is generally used because it enables multi-foci generation to stimulate targeted neural ensembles simultaneously.”
But, as Kong points out, opsins (akin to tiny solar cells) are on cell membranes. To ensure efficient stimulation, enough opsins need to be activated to generate action potentials.
“In fact, the size of neurons within a mouse cortex ranges from 10 to 20 µm, much beyond the laser focus size of conventional two-photon systems,” he says. “In earlier efforts, extended laser foci, such as disk patterns, were designed to match the size of neurons. No doubt extended stimulation patterns are generally of low axial resolutions, which would lead to mis-stimulation. Moreover, the speckles in extended patterns decrease stimulation efficiency.”
Optics involved
Two-photon optogenetics achieves excitation or inhibition of neural activity in neuroscience based on a nonlinear optical process, two-photon excitation (2PE). “Compared to one-photon excitation-based optogenetics, two-photon optogenetics can achieve precise stimulation within deep tissues, thanks to the inherent optical-sectioning capability of 2PE and the increased robustness to scattering of the longer-operation wavelengths,” points out Kong.
CGH is a technique to obtain a complex optical field via numerical calculation. “For two-photon optogenetics based on CGH, you calculate a hologram pattern based on spatial distributions of targeted neural ensembles, via computer, then display the hologram pattern onto a spatial light modulator for selective stimulation,” he says.
And all-optical physiology is an integrated optical technique for stimulating and recording neuron activities simultaneously. “In our proposed system, two-photon fluorescence imaging and two-photon optogenetics are integrated, which enables deep tissue imaging and stimulation of neural activities in vivo, at high spatio-temporal resolutions,” Kong adds.
Commonly used extended stimulation patterns for two-photon optogenetics fail to achieve single-neuron resolution—particularly within the axial dimension, where inherent speckles decrease stimulation efficiency.
A quick fix
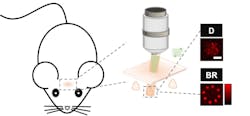
Instead of using complex techniques to improve axial resolution or eliminate speckles, “we propose using speckle-free beaded-ring patterns for two-photon optogenetics,” says Kong. “It achieves both high axial resolution and high stimulation efficiency. We demonstrated its superior advantages via two-photon optogenetics based on all-optical physiology within a mouse’s primary somatosensory cortex in vivo.” (See figure.)The most surprising aspect of this work was the discovery of an inexpensive alternative way to get around shortcomings in current two-photon optogenetics by simply changing the phase patterns displayed within any existing CGH-based two-photon optogenetics systems—without making any other changes. “It’s a good substitute for existing technologies,” Kong says.
With advanced techniques available now, Kong and colleagues are working toward all-optical physiology for simultaneously optical recording and manipulating neural activities across cortical layers or areas in vivo. “Our method will enable the study of long-range functional connectivity for the first time,” he adds.

Sally Cole Johnson | Editor in Chief
Sally Cole Johnson, Laser Focus World’s editor in chief, is a science and technology journalist who specializes in physics and semiconductors. She wrote for the American Institute of Physics for more than 15 years, complexity for the Santa Fe Institute, and theoretical physics and neuroscience for the Kavli Foundation.