Remote Sensing: Laser spectroscopy—Using active FTIR to distinguish between multiple gases
CHRISTOPHER G. LEBURN, OGUZHAN KARA, and DERRYCK T. REID
Fugitive hydrocarbon emissions are estimated to cost the energy sector $5 billion per year and account for 12% of greenhouse gas emissions—they also are thought to jeopardize safety and public health, as well as act as a key factor in climate change. Whether to enable industrial sites close to communities to conduct 24-hour continuous sensing, or to assess efficiencies in combustion engines, markets including oil and gas, landfill, and agriculture are driving a growing requirement for high-resolution gas-detection solutions that are compact, portable, and affordable.
Sensing different gases can be achieved in different ways. Differential absorption lidar (DIAL) is considered to be one of the most advanced techniques. With a range of 500 m, this technique directs high-energy laser light into the atmosphere, which is returned to a ground-based detector by weak scattering from airborne particles. Unfortunately, DIAL systems are complex, costly to run, and very large, with some systems being housed on an 18-wheeler truck.
By contrast, Fourier-transform infrared (FTIR) spectroscopy is naturally broadband and offers far wider coverage than DIAL. In an open-path setup, FTIR has the capability to detect hundreds of atmospheric gases and, with a small system footprint comparable to a briefcase, is much more portable than a DIAL system. Open-path FTIR typically uses thermal sources to quantify hydrocarbon emissions, but with typical commercial solutions having resolutions of 0.5 cm-1, it is difficult to separate out multiple species when they are spectrally overlapped. In addition, in-the-field FTIR spectroscopy using thermal sources usually requires high-quality retroreflecting targets to direct the source light back towards the detector.
Laser-based active FTIR spectroscopy offers higher resolution, providing the capability to distinguish similar gases such as methane and ethane. But to achieve this in the atmosphere, a broadband light source is required with a brightness high enough to be effective over long distances—such a light source is not currently available on the market. Quantum-cascade laser (QCL) technologies have been used for stand-off detection measurements of water vapor, methane, nitrous oxide, and hydrogen peroxide. However, while certainly a cheaper alternative than DIAL, QCLs provide only a narrow linewidth, which limits their capability when it comes to the detection of multiple species.
Researchers at Heriot-Watt University have been working with ultrafast laser manufacturer Chromacity (both in Edinburgh, Scotland) to develop an eye-safe active FTIR spectroscopy system capable of acquiring sub-0.1-cm-1 resolution gas absorption spectra from simple targets at ranges exceeding 70 m. This is a first step towards a real-time solution for simultaneously quantifying multiple gases across several hundred meters.
The gas-sensing system
The setup is broken into three main elements: the broadband light source, the spectrometer (consisting of interferometer and detection system), and a computer algorithm to extract the data.
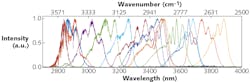
The light source was the Chromacity broadband, ultrafast optical parametric oscillator (OPO). This particular model was optimized to provide tunability from 2800 to 3900 nm, so that the strongest absorptions in methane and ethane (3.1–3.5 μm) could be detected and identified (see Fig. 1). This high-brightness source delivered average powers greater than 300 mW—a 1-cm-diameter beam was passed into the spectrometer.
Light from the Chromacity OPO was first coupled into a scanning Michelson interferometer before being launched into free space (see Fig. 2). The scattered signal returned from the target was subsequently collected by a 6-in. f/4 Newtonian telescope and detected using an indium antimonide (InSb) liquid-nitrogen-cooled photodiode. Light from the OPO was launched along an optical axis co-aligned with the telescope’s field of view using a small 45° steering mirror situated directly before the secondary mirror of the telescope. The scanning interferometer operated at 1 Hz and achieved a typical resolution of 0.05 cm-1, which is sufficient to resolve the narrow and complex absorption-line structure of light molecules like water, methane, and ethane.
To deconvolve the information received from the spectrometer, a patent-pending computer algorithm was developed. Quantitative open-path spectroscopy requires either a reliable reference spectrum or a method of inferring the original illumination spectrum, and this problem has been treated in different ways. The approach used here resulted in the retrieval of an illumination spectrum, which represents the OPO output spectrum prior to undergoing atmospheric absorption.
Multi-species measurements at 30 m range
The first series of experiments was designed to establish the ability to measure multiple spectrally overlapping species simultaneously. Indoor measurements were performed using a rough aluminum-foil target placed at a range of up to 30 m from the FTIR system. Light launched from the OPO entered a 20-cm-long gas cell containing a 1.5±0.15% ethane-in-air mixture. This cell was situated directly in front of the secondary mirror of the telescope.
Figure 3 gives an example of a single measured spectrum (no averaging) exhibiting densely packed absorption lines from water, methane, and ethane, as well as continuum absorption from ethane, which suppresses the overall spectral intensity.
The algorithm retrieves the effective illumination spectrum (see Fig. 3a, dashed line), which represents the OPO output spectrum prior to undergoing atmospheric absorption. The black line in Figure 3a is the best-fit absorption spectrum using 0.1-cm-1-resolution Pacific Northwest National Laboratory (PNNL; Richland, WA) database data as the fitting reference. The inset in Figure 3a shows the typical correspondence between the measured spectrum and the best-fit data. Figure 3b shows the extracted concentration data for water, methane, and ethane.
These experiments showed that the system was able to obtain environmental concentration values consistent with independent humidity measurements (water), established ambient levels (methane), or known control concentrations (ethane).
Real-time methane emission measurement at 70 m range
The next phase of the project extended the range of detection and also performed a real-time measurement of a simulated gas leak. A 78 m corridor was used at the test site. A 2% methane:air mix was released for 100 s at a rate of 103 µg s-1 at a distance of 65 m from the Chromacity OPO. During this experiment, the ethane cell was not present. Spectra were recorded every seven seconds and fitted in the same way as the previous experiment to provide concentrations of water, methane, and ethane.
Figure 4a shows an example of a spectrum recorded without averaging at 70 m range and at a moment close to the peak methane emission. The inset of Figure 4a shows the correspondence to the best-fit PNNL database across the 3.18–3.21 μm region. In contrast to ethane, methane and water show very little continuum absorption under these experimental conditions (20°C, 101,800 Pa), so the inferred illumination spectrum closely follows the envelope of the measured spectrum. Figure 4b presents the measured water and methane concentrations over 400 s, showing the methane concentration rising from background levels (around 1900 ppb) to a peak of around 13,000 ppb before returning to near the original value as the gas disperses. Prior to the methane release, the root mean squared (RMS) variation of the measured concentration of background methane at this range was <100 ppb. Water showed more variability, which is expected to be associated with convection effects and environmental variations.
This system offers several advantages over previously reported open-path sensing of atmospheric species. The measurements conducted here were carried out with very simple, alignment-free targets, typically a coarsely positioned sheet of aluminum foil at a range of up to 70 m, but also with returns from >30 m available from paper, laminate, or similar targets.
Emission measurement beyond 70 m
During these experiments, the lengths of the measurements have been constrained by the dimensions of the available buildings. Based on the high brightness of the Chromacity OPO, the signal-to-noise performance clearly indicates that measurements beyond 70 m will be possible with no modification to the current setup.
The ability to use a simple topographic target increases the practicality of the system. As there is no need to maintain the beam on a small retroreflector, the beam-pointing accuracy needed is reduced and, in some cases, eliminates the need for a remote target. In a recent outdoor campaign, the team extended measurements at a 3.5 µm wavelength to more than 200 m, using only the aluminum tailgate of a truck as the retroreflecting target.
A highlight of this system’s performance is its ability to extract concentration data from a single spectrum with no need for averaging, which provides a real-time and quantitative monitoring capability and makes the system suitable for detecting weak, localized emissions.
The resolution of 0.05 cm-1 shows that it is possible to extract concentration measurements from complex, spectrally overlapping multiple species. Combined with the real-time monitoring capabilities of the system, this feature could permit the observation of correlations between different hydrocarbon gas concentrations, providing insights into their origins. For example, separate petrochemical methane contributions (accompanied by weak ethane signatures) can be separated from biogenic sources (such as cattle, landfill, etc.), which produce only methane.
The entire combined system, including the Chromacity OPO and spectrometer, fits on a 60 × 90 cm breadboard, making it relatively easy to transport. The system also has the ability to quantify many other chemical signatures. These features open up significant opportunities for the development of a cost-effective and highly portable environmental multi-gas-sensing solution.
Christopher G. Leburn is at Chromacity and Oguzhan Kara and Derryck T. Reid are at Heriot-Watt University, all in Edinburgh, Scotland; e-mail: [email protected]; www.chromacitylasers.com.